
Fig.1 Changes in peel color of GP-DOV
KAN Jianquan 1,2, CHEN Kewei 1, ZHANG Fusheng 1,2, ZHENG Jiong 1,2
(1. College of Food Science, Southwest University, Chongqing 400715, China; 2. Laboratory of Quality and Safety Risk Assessment for Agro-products on Storage and Preservation (Chongqing), Ministry of Agriculture, Chongqing 400715, China)
Abstract:Fast drying could restrain chlorophyll (Chl) degradation in green prickleyashes (GPs) drying except for GPs with damaged oil vacuoles (GP-DOV). To explore the possible mechanisms behind this phenomenon, the variations in Chl pigments and related enzymes were investigated. Compared with GPs with intact oil vacuole, the variations in Chl pigments in GP-DOV were more serious and complicated, that is, only 6% of the total Chl was retained in GP-DOV. This damage seemed to inhibit the formation of pyrochlorophyll pigments after the formation of pheophorbide-a in the pheophorbide-a oxygenase pathway and resulted in the accumulation of pheophorbide a, which accounted for 18% of the total Chl pigments in dried GP. During the slow drying process, the damage gradually induced the activity of chlorophyll-degrading peroxidase, resulting in the accumulation of C13 2oxidized Chl pigments. The activities of related enzymes such as chlorophyllase and pheophorbidase showed a downward trend whereas chlorophyll-degrading peroxidase and metal-chelating substances was fl uctuated.
Key words:green prickleyash; drying; chlorophyll breakdown
The green prickleyash (GP) Zanthoxylum schinifolium Zucc., characterized by a strong spicy and astringent taste, is a traditional spice found in China and other subtropical areas. Its fruit presents a bright green color because of the high content of chlorophyll (Chl) pigments in the peel. Dried GPs are the main products for commercial trade [1]. During harvest seasons, when farmers pick bunches of GPs from the trees and place them on the ground to dry under the sun, oil vacuoles should be given extra care because they are vulnerable to damage. Mechanical destruction of oil vacuoles causes quick degradation of the greenness of GPs, resulting in an undesirable brownness that greatly affects its marketability. “Brown prickleyashes” have poor quality and sold at a very low price.
Chl pigments are responsible for the typical green color of vegetables and fruits. They are also easy todegrade into colorless compounds during leaf senescence and fruit ripening. A study reported that Chl-a follows the pheophorbide (Pheide)-a oxygenase (PAO) degradation pathway to become non-colored linear tetrapyrroles [2]. The reaction is initiated by converting Chl-a to chlorophyllide (Chlide)-a using chlorophyllase (Chlase) and then removing magnesium from the porphyrin ring of Chlide-a to generate pheophorbide (Pheide)-a in the presence of a metal-chelating substance (MCS), which may be non-enzymatic in nature [3-5]. The conversion of Pheide-a to primary fluorescent Chl catabolites (FCCs) requires two enzymes: PAO and red chlorophyll catabolite reductase. Finally, FCCs undergo a series of modifications to yield colorless non-fluorescent Chl catabolites [2,6]. Pheide-a can also be converted to pyropheophorbide (PyroPheide)-a, another methoxycarbonylfree Chl catabolite, through chemical and physical modifications [7-8]. Pheophorbidase (Phedase) is responsible for the conversion of demethylated Pheide-a to C13 2-carboxyl PyroPheide-a. C13 2-carboxyl PyroPheide-a then changes to PyroPheide-a by non-enzymatic decarboxylation. Pheophorbide demethoxycarbonylase (PDC), another enzyme found in some Chl-b-less mutants, is identified to directly catalyze Pheide-a to PyroPheide-a without any intermediate [9].
Several studies reported that Chl-a can undergo another conversion to form C13 2-hydroxychlorophyll (C13 2-OH Chl)-a in the storage of some green vegetables [10-13]. This conversion is mainly mediated by chlorophyll-degrading peroxidase (Chl-POD), which facilitates the oxidization of Chl-a at C13 2in the presence of polyphenols and hydrogen peroxide [14]. Except for common Chl catabolites, several other types of Chl derivatives, such as C15 1-hydroxy-lactone chlorophyll (C15 1-OH-lactone Chl)-a, pyrochlorophyllide (PyroChlide)-a, and pyropheophytin (PyroPhy)-a, were also found in some ripen fruits or processed food [15-16].
However, only a few studies reported on the color changes and chlorophyll degradation in the post-harvest processing of GPs. In our previous study, the chlorophyll breakdown was initiated after the fruits were detached from the tree, especially during slow drying where GPs were placed in the shade to dry. Considering that oil vacuoles are intact, Chl-a and Chl-b are the major Chl pigments in GPs before and after slow drying [1]. However, the results showed that these two types of Chl pigments minimally contribute in dried GPs with damaged oil vacuoles (GP-DOV). In some extreme cases, dried GP-DOV is totally black, which is much worse than slow-dried GPs with intact oil vacuoles (GP-IOV). This study examines the mechanisms behind Chl degradation in GP-DOV drying, including the changes in peel color, enzyme activity, and content of chlorophylls and their derivatives. The relationships among these changes were also established to explore the possible mechanisms behind color degradation during GP-DOV drying.
1.1 Raw materials
Fresh GPs were harvested at commercial maturity from a local farm in Chongqing, China. They were picked carefully from the same tree to ensure consistency. Leafstalks were cut off to leave only subglobose fruits. The broken oil vacuoles were induced on an oscillator, and 50 g fresh GPs in a 500 mL Erlenmeyer fl ask were treated at 200 r/min for 60 s. Two drying modes (slow and fast) were employed to dry GP-DOV. To avoid the interference of moisture content, 5 g fresh GP-DOV was placed in a polyvinyl plastic opentank before drying. Slow-dried samples were placed indoors in dim light to evaporate water, where an environment of 25 ℃ with 50% relative humidity was maintained by an airconditioner. Fast-dried samples were dried in an oven at 50 ℃to obtain greenish products from intact oil vacuoles before drying. Samples were determined at an interval of 12 h for slow drying and 1 h for fast drying until the moisture content dropped below 5%. Finally, the products were obtained after 108 h in slow mode and 10 h in fast mode. The samples were frozen at -80 ℃ before analysis.
1.2 Determining color and moisture content
An Ultra Scan PRO (Hunterlab, America) was applied to evaluate the color changes in GPs peel. The procedure was repeated 20 times to minimize inaccuracy. The samples were dried in an oven at 100 ℃ to constant weight to measure moisture content.
1.3 Acetone powder preparation
Acetone powder of GPs was prepared according to the methods described by Roca et al. [15]. Deseeded GPs were homogenized with 20 volumes of cold acetone (-20 ℃), and the supernatant was fi ltrated and discarded. The residue was continually washed with 5 volumes of cold acetone to achieve a colorless percolate. Finally, the residue was dried by cold wind to obtain dry powder. Approximately 0.12 g acetone powder was obtained from 1 g deseeded GPs (fresh weight).
1.4 Enzyme extraction
The crude enzyme was extracted from acetone powderusing the method described by Fukasawa et al. [17]. Briefly, 500 mg acetone powder was treated with 15 mL of sodium phosphate buffer (10 mmol/L, pH 7.0) to extract Chl-POD and then with 15 mL of sodium phosphate buffer (50 mmol/L, pH 7.0) containing 50 mmol/L potassium chloride and 0.24 g/100 mL Triton-X-100 to obtain Chlase, Phedase, and MCS. This procedure was carried out in an ice bath with 1 h stirring. The supernatant, applied as the crude enzyme extract, was acquired by fi ltration through four layers of cotton gauze and subsequent centrifugation (Sigma, 4K15, Germany) at 9 000 × g for 15 min at 4 ℃.
1.5 Pigment preparation and analysis
1.5.1 Standard pigment preparation
Chl-a and Chl-b extracted from spinach leaves were employed to prepare standard pigments [1]. Dephytoled Chl derivates (Chlide-a and Pheide-a) were acquired from their corresponding Chl parents by enzymatic deesterifi cation using a Chlase partially purifi ed (20% to 40% of ammonium sulfate) from citrus peels [18]. C13 2-OH Chl pigments were prepared by selenium dioxide oxidation of their corresponding Chl pigments with reflux-heating for 4 h in pyridine solution under argon. Carbomethoxy-free Chl derivatives (pyropheophytin, PyroChlide, and PyroPheide) were also prepared from their respective parents by refluxheating at 100 ℃ in collidine [15]. C15 1-OH-lacton Chl was obtained by oxidation in alkaline medium achieved by mixing Chl acetone solution with 0.5% sodium hydroxide [19]. Sequentially, Chl was oxidized by atmospheric oxygen after stirring at room temperature for 10 min. The above oxidized products were transferred to diethyl ether and washed with sodium chloride (NaCl)-saturated solution. The separation of C13 2-OH Chl and C15 1-OH-lacton Chl from mixtures was carried out by thin-layer chromatography and sequent preparative high-performance liquid chromatography (HPLC, Shimadzu, Japan). All magnesium-free Chl derivatives were acquired from their corresponding Chl precursors in diethyl ether using acidifi cation by adding four to fi ve drops of 10% hydrochloric acid and then washed with distilled water.
1.5.2 Sample pigments analysis
Pigments were extracted by the method described by Roca et al. [15]with some modifications. The samples (5 g in fresh weight) were deseeded before homogenization in 30 mL dimethylformamide (DMF) saturated with magnesium carbonate, and the residue was fi ltrated and washed with DMF repeatedly until the fi ltrates were colorless. The combination of the DMF extracts was washed thrice with 50 mL hexane to eliminate lipids and oils, and the Chl pigments were reserved in DMF phase. Subsequently, 10 g/100 mL NaCl solution (0 ℃) was added into the DMF phase, and diethyl etherhexane (1:1, V/V) was applied to extract the Chl pigments from the DMF aqueous phase. This extraction was repeated until the aqueous phase became colorless. The combined organic phase was continuously washed with distilled water to eliminate polyphenols and other water-soluble compounds and then dehydrated with anhydrous sodium sulfate. The organic phase was evaporated under vacuum below 30 ℃, and the dryness was dissolved in 5.0 mL acetone.
Separation was performed on a GL Sciences column (ODS-SP, 4.6 mm × 250 mm, 5 μm) with a two-mobile-phase eluting system following the method described by Roca et al. [15]: (A) ion pair reagent/water/methanol (1:1:8) and (B) methanol/acetone (1:1). The ion pair reagent was 0.05 mol/L tetrabutyl ammonium bromide and 1 mol/L ammonium acetate in water. The eluting program was operated as follows: the ratio of B/A mobile phase increased linearly from 0% to 100% within 35 min. Isocratic B was held for 15 min to stop the eluting procedure, and mobile phase A was held for another 10 min to condition the column before the next injection. The fl ow rate was kept at 1 mL/min throughout the separation, apart from the period from 30 min to 50 min, which had a low fl ow rate at 0.6 mL/min. The Chl pigments and their derivatives were determined by HPLC according to the method described by Fraser and Frankl [20].
1.6 Measurement of chlorophyllase, peroxidase, pheophorbidase, and MCS dechelating activity
The enzyme activity involved in Chl degradation was measured through HPLC. For Chlase, the method was described by Yang et al. [21]. The reaction mixture contained 500 μL of 0.1 mol/L phosphate buffer (pH 7.5) with 0.15% Triton-X-100, 100 μL of Chl-a (500 μg/mL) acetone solution, and 500 μL crude enzyme extract. As for Chl-POD, the method was described by Yamauchi et al. [22]with some modifi cations. The standard reaction system (1 500 μL) contained 750 μL of 0.1 mmol/L phosphate buffer (pH 5.5), 50 μL of 1.0 g/100 mL Triton-X 100, 50 μL of 5 mmol/L p-coumaric acid in ethanol solution, 50 μL of 0.3% hydrogen peroxide, 100 μL of Chl-a (500 μg/mL) acetone solution, and 500 μL crude enzyme extract. Both reaction mixtures were incubated at 25 ℃ for 60 min in dim light. The reactions were stopped by adding 5 mL acetone for Chlase and 6 mL for Chl-POD. The assay of Phedase activity was carried out following the method described by Suzuki et al. [9]with some modifications. Thereaction mixture in a total volume of 800 μL consisted of 20 mmol/L phosphate buffer (pH 7.0) with 4.9 μg Pheide-a and 200 μL crude enzyme extract. The mixture was incubated at 25 ℃ for 2 h to finish the spontaneous decarboxylation. The reaction was stopped by adding 3.2 mL acetone. The samples were stored at -20 ℃ for approximately 18 h prior to HPLC. To measure MCS dechelating activity, the reaction mixture with 800 μL of 10 mmol/L phosphate buffer (pH 7.5), 250 μL of Chlide-a (6.2 μg) in water, and 200 μL crude enzyme extract was incubated at 37 ℃ and then immediately analyzed by HPLC. One unit of Chlase, Chl-POD, Phedase, and MCS activity was defined as the formation of 1 μg of Chlide-a, C13 2-OH-Chl-a, PyroPheide-a, and Pheide-a per min, respectively. Protein content was assayed by the method described by Brandford [23]using bovine serum albumin as standard. Each measurement was performed in triplicate.
1.7 Statistical analysis
GPs were selected randomly, and each sample was determined thrice, except for the color measurement, which was determined 20 times. Analysis of variance was performed using Duncan’s test from SAS 6.0 statistical software package with signifi cance at the 0.05 level. All results are represented as mean ± standard deviation (SD).
2.1 Peel color and Chl content
The values of H* and a* were recorded to monitor color changes in the peels of GP-DOV. The a* value, correlating the green color of peel, increased rapidly from below -2 to over 4 in the two drying modes (Fig.1). This result agreed with our visual observation that the green color degraded quickly after oil vacuoles were destroyed. The fi nal products dried at 50 ℃ showed a slightly lower a* value than those dried at 25 ℃ but still showed an undesirable degreening. However, the H* value showed a sharply decreasing trend. Moreover, the final products showed no conspicuous difference between the two drying conditions. Compared with the samples of intact oil vacuoles whose green color can be retained by fast drying [1], the destruction of oil vacuoles prompted a rapid degradation in color, i.e., conversion from a typical green to an undesirable brown in a short time.
The associated Chl changes were also coincidental with the degreening process. Chl variations in the two drying modes were compared in Fig. 2. At 50 ℃, Ch1-a and Chl-b decreased by approximately 88% and 76%, respectively, with the total Chl content descending from 210.50 μg/g FW to 31.49 μg/g FW. This Chl degradation can explain why fast drying could not retain the green color when oil vacuoles were damaged. At 25 ℃, a larger amount of Chl was degraded, where Ch1-a and Chl-b declined by approximately 97% and 86%, respectively, with only 13.06 μg/g FW of the total Chl left in the dried GP. This decomposition proportion of Chl in GP-DOV (94%) is more than two times higher than that in GP-IOV (44% in the total Chl, data not shown) under the same drying condition (25 ℃). The degradation rate in GP-DOV was also significant. Approximately 73% and 78% of the total Chl-a were decomposed at 50 ℃ and 25 ℃ within 3 h and 24 h, respectively. However, no signifi cant difference in Chl content was observed between the dried GPDOVs acquired from different drying temperatures. Fast drying failed to limit Chl degradation in GP-DOV.

Fig.1 Changes in peel color of GP-DOV
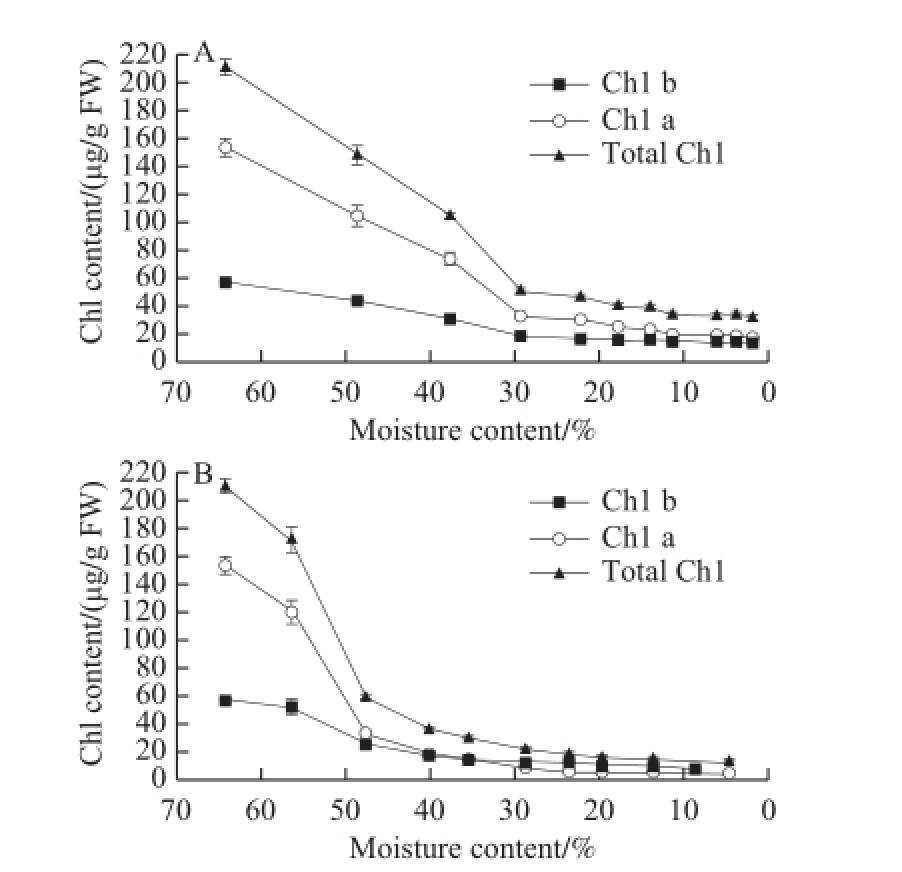
Fig.2 Changes in chlorophyll contents at 50 ℃ (A) and 25 ℃(B)
2.2 Chl derivatives and their related enzyme activities
As shown in Table 1, the pigments in the peel of GP-DOV were separated and identified according to the comparison of the retention time and spectrum with the standard Chl pigments and their derivatives. The resulting Chl derivatives in GP-DOV were much more diverse than those in GP-IOV. Changes in enzyme activity and Chl-related derivatives throughout the drying process were illustrated in Fig.3 and Fig.4, respectively.
Table 1 Chromatographic and spectroscopic characteristics of chlorophylls and their derivatives in GP-DOV
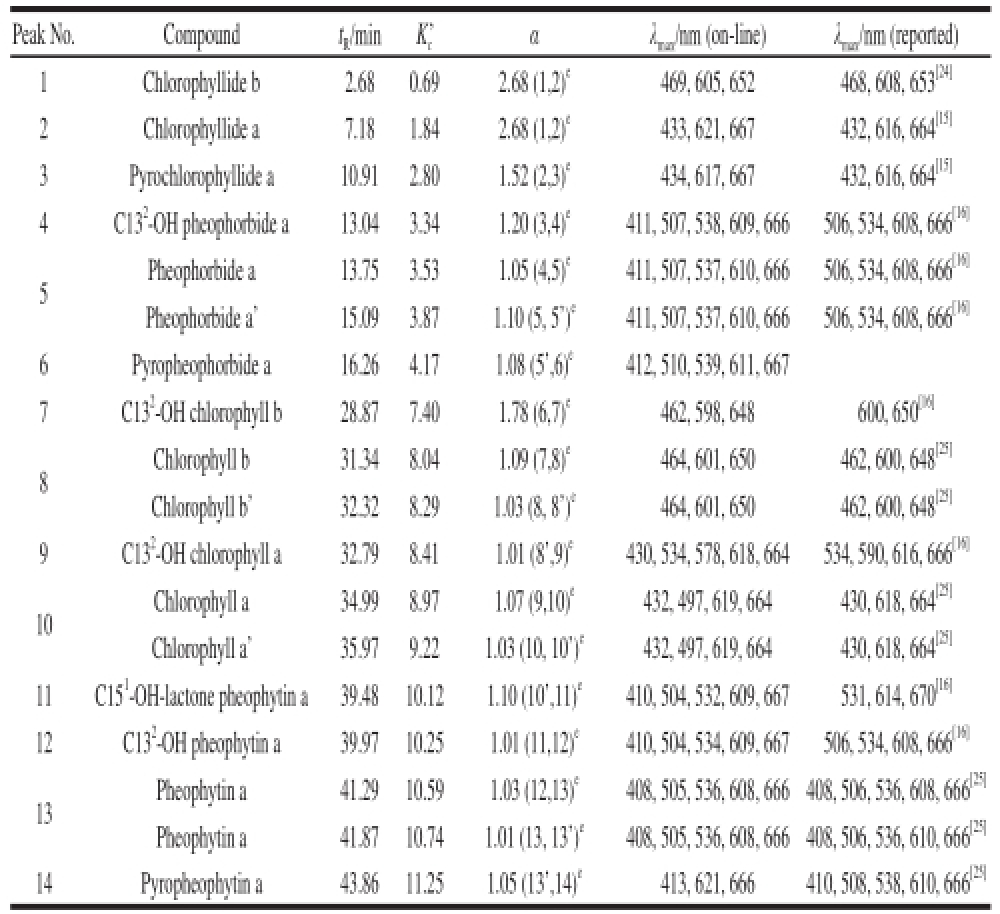
Note: t R=t r-t 0,where t ris the retention time of pigment peak and t 0is the retention time of unrestrained component; K c’ and α represent capacity factor and separation factor respectively; λ max. Maximum absorbance wavelength; e. Numbers in parentheses stand for the two neighboring peaks.
Peak No.Compoundt R/minK c’αλ max/nm (on-line)λ max/nm (reported) 1Chlorophyllide b2.680.692.68 (1,2) e469, 605, 652468, 608, 653 [24]2Chlorophyllide a7.181.842.68 (1,2) e433, 621, 667432, 616, 664 [15]3Pyrochlorophyllide a10.912.801.52 (2,3) e434, 617, 667432, 616, 664 [15]4C13 2-OH pheophorbide a13.043.341.20 (3,4) e411, 507, 538, 609, 666506, 534, 608, 666 [16]5Pheophorbide a13.753.531.05 (4,5) e411, 507, 537, 610, 666506, 534, 608, 666 [16]Pheophorbide a’15.093.871.10 (5, 5’) e411, 507, 537, 610, 666506, 534, 608, 666 [16]6Pyropheophorbide a16.264.171.08 (5’,6) e412, 510, 539, 611, 667 7C13 2-OH chlorophyll b28.877.401.78 (6,7) e462, 598, 648600, 650 [16]8 Chlorophyll b31.348.041.09 (7,8) e464, 601, 650462, 600, 648 [25]Chlorophyll b’32.328.291.03 (8, 8’) e464, 601, 650462, 600, 648 [25]9C13 2-OH chlorophyll a32.798.411.01 (8’,9) e430, 534, 578, 618, 664534, 590, 616, 666 [16]10 Chlorophyll a34.998.971.07 (9,10) e432, 497, 619, 664430, 618, 664 [25]Chlorophyll a’35.979.221.03 (10, 10’) e432, 497, 619, 664430, 618, 664 [25]11C15 1-OH-lactone pheophytin a39.4810.121.10 (10’,11) e410, 504, 532, 609, 667531, 614, 670 [16]12C13 2-OH pheophytin a39.9710.251.01 (11,12) e410, 504, 534, 609, 667506, 534, 608, 666 [16]13Pheophytin a41.2910.591.03 (12,13) e408, 505, 536, 608, 666 408, 506, 536, 608, 666 [25]Pheophytin a41.8710.741.01 (13, 13’) e408, 505, 536, 608, 666 408, 506, 536, 610, 666 [25]14Pyropheophytin a43.8611.251.05 (13’,14) e413, 621, 666410, 508, 538, 610, 666 [25]
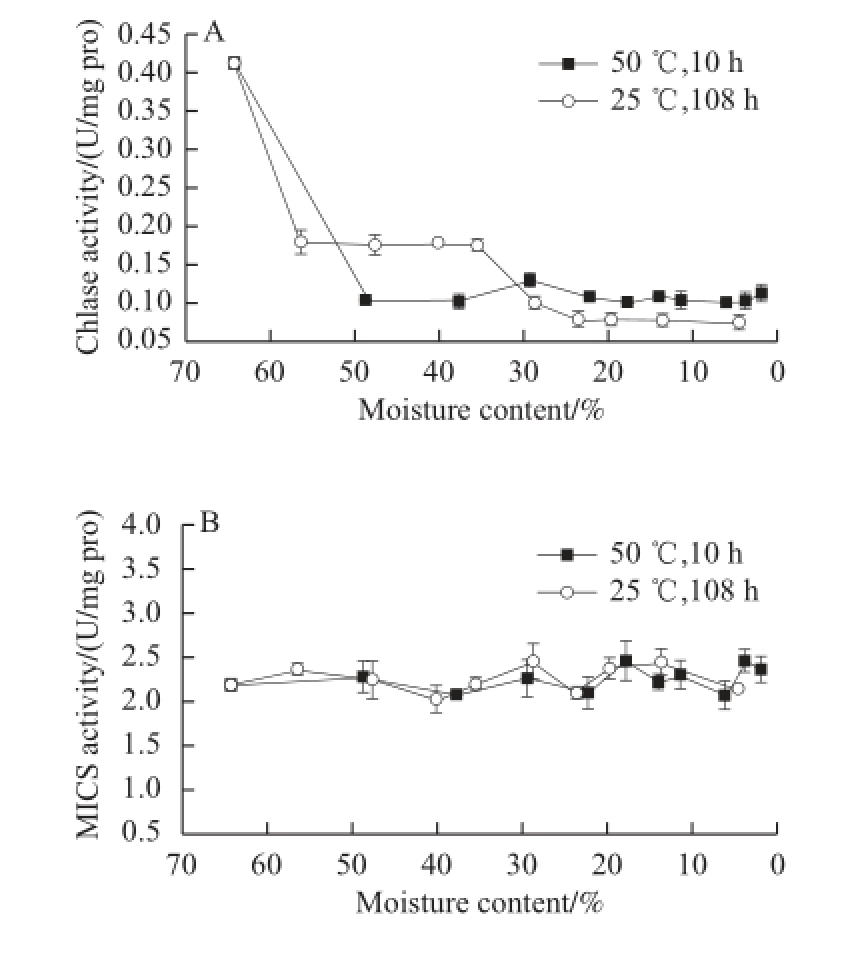
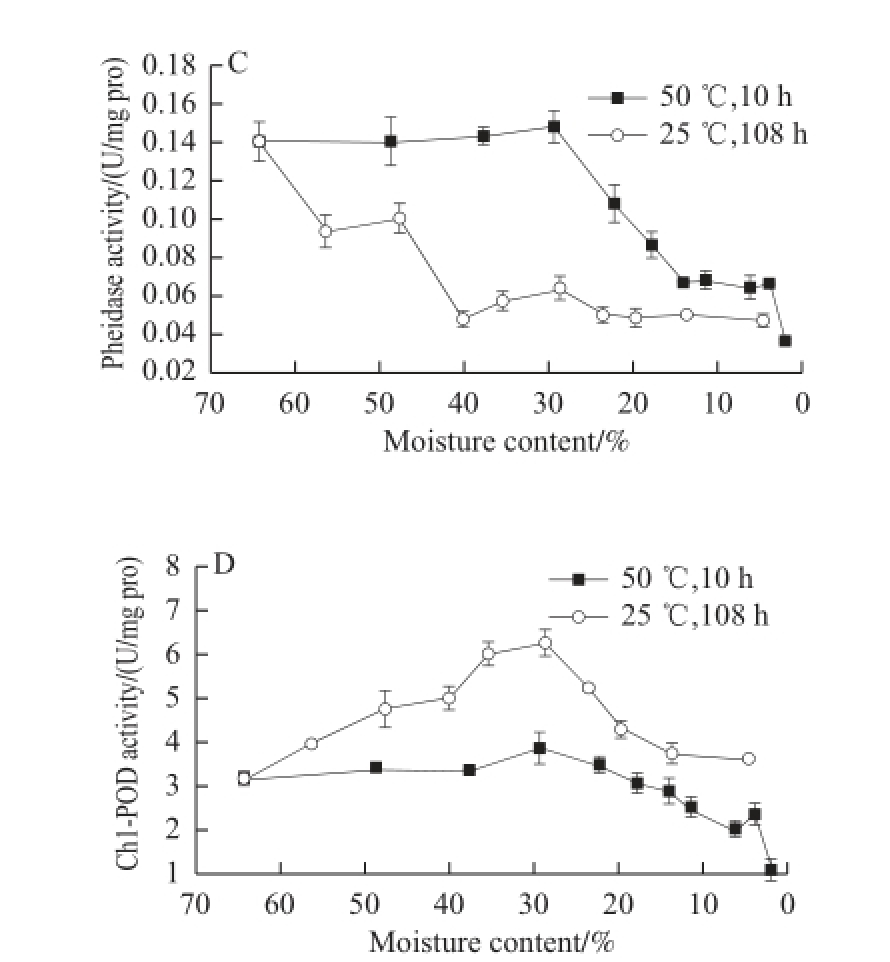
Fig.3 Changes in enzyme activities involved in Chl degradation and in Mg-dechalating activity in GP-DOV
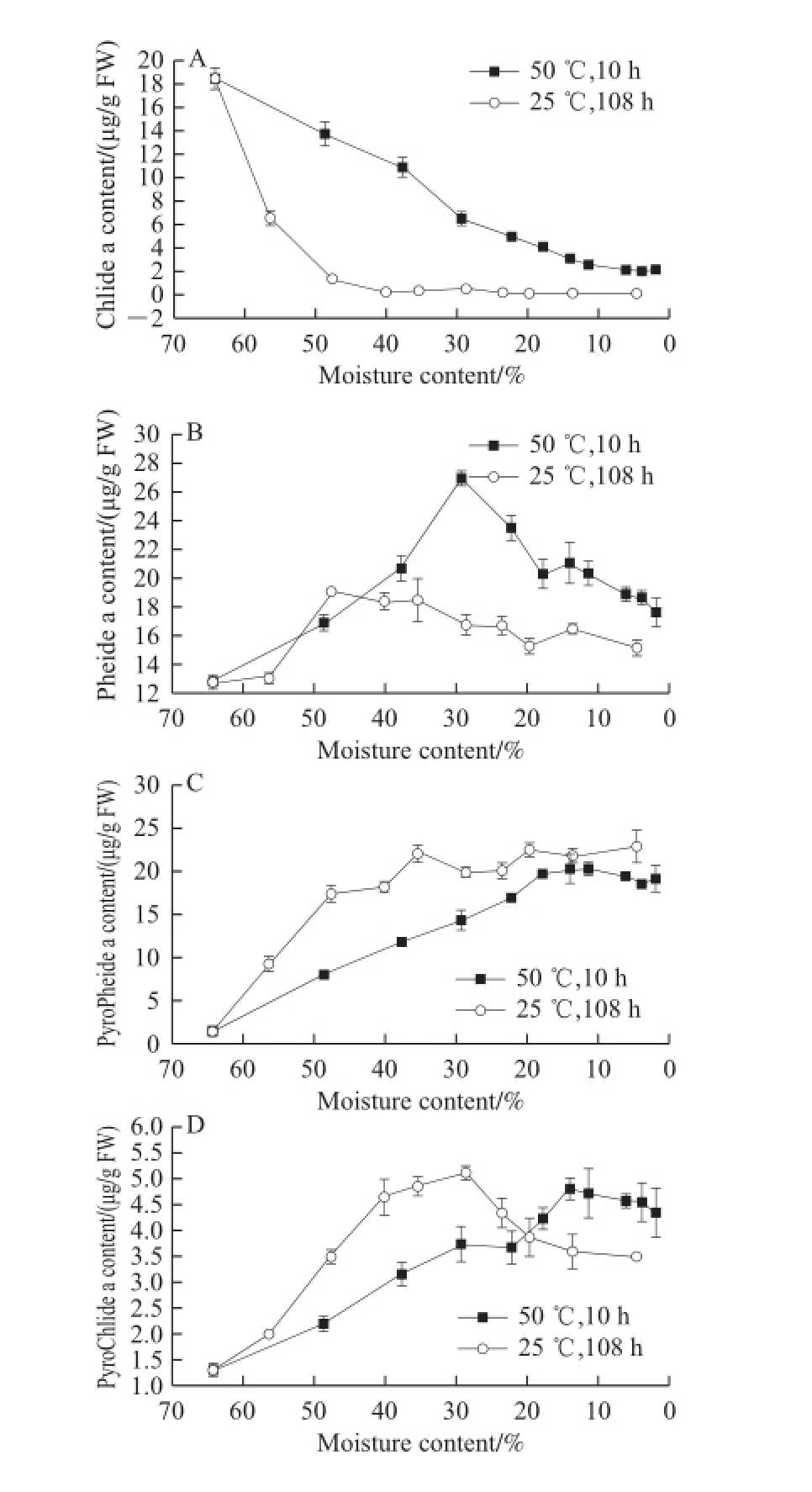
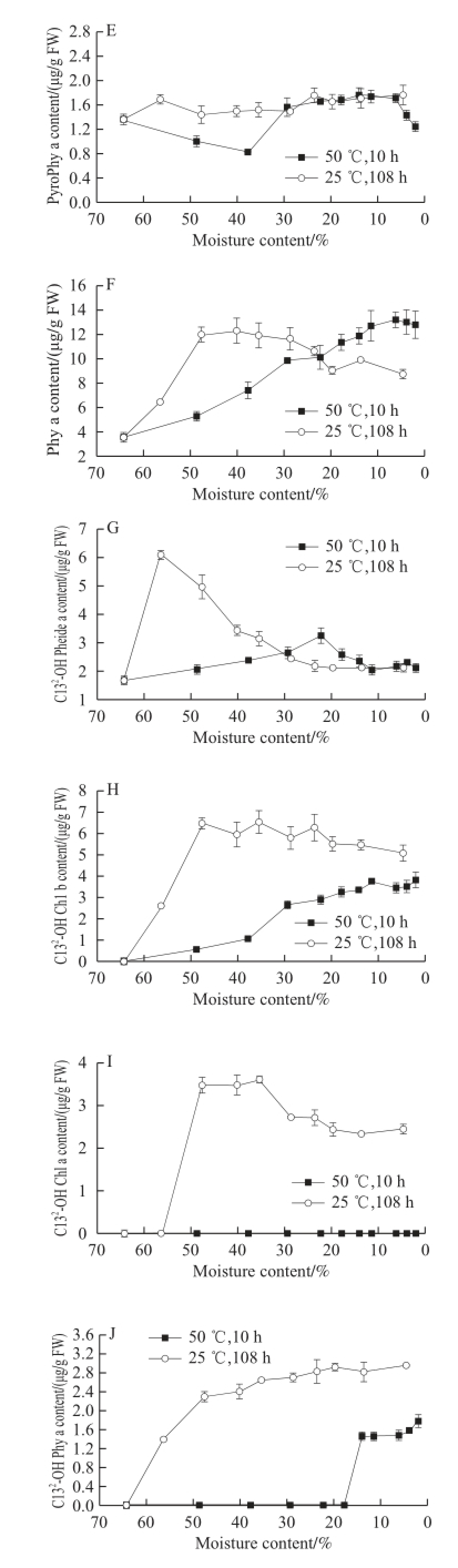

Fig.4 Changes in chlorophyll derivatives in GP-DOV
2.2.1 Changes in Chlase, MCS, and their associated Chl derivatives
As shown in Fig. 4A, Chlase showed similar patterns in both drying modes, which corresponded with other findings that reported a reducing activity when leaf senescence was initiated [18,21]. This activity change was parallel with the changes in Chlide-a, the product of Chlase reaction, which occupied the majority (18.48 μg/g FW) of Chl derivatives at the beginning of drying but declined (<3 μg/g FW) in dried GPs under both drying conditions.
The sharp decrease in Chlide-a may also be attributed to the consistent level of MCS activity (Fig. 3B), reflecting its non-enzymatic origin and non-inducement in aging [3,5]. As shown in Fig.4B, the high drying temperature did not affect MCS activity, and the stable MCS continuously dechelated magnesium from Chlide-a to yield Pheide-a, which substantially accumulated in both drying modes, culminating at 26.91 μg/g FW within 3 h for fast drying and at 19.04 μg/g FW within 24 h for slow drying. Pheide-a was diminished in the following drying but was left at high levels in the dried GPs.
2.2.2 Changes in Phedase and its associated Chl derivatives
Although it remained almost constant in the first 3 h of drying at 50 ℃, Phedase (Fig. 3C) that facilitated the removal of carbomethoxy showed a downward trend in the drying process.
However, the formation of carbomethoxy-free Chl pigments was diverse. The majority of pyrochlorophyll pigments belonged to PyroPhiede-a (Fig. 4C). In the 25 ℃ and 50 ℃drying modes, its content increased dramatically from less than 3 μg/g FW to approximately 20 μg/g FW, which consisted most of the Chl pigments in the dried GP-DOV. As shown in Fig. 4D, PyroChlide-a showed an upward trend in the drying process. The content of PyroPhy-a (Fig. 4E), which was not detected in GP-IOV drying fluctuated in a trace amount throughout the drying process [1]. The appearance of carbomethoxy-free Chl may be attributed to the enzyme reactions.
Several studies reported that PyroPhiede-a can be induced by Pheidase from Pheide-a. Moreover, other decarbomethoxy Chl derivatives (PyroChlide-a and PyroPhy-a) can be catalyzed in a similar way by Pheidase [9]. Our previous study also confi rmed the fi nding that Pheidase is involved in decarbomethoxy reactions [1]. The destruction of oil vacuoles increased the accessibility of enzymes and substrates. This structural change benefited Pheidase reactions, which are also helpful for pyrochlorophyll accumulation. Pheidase had a relatively high activity before 3 h in fast drying or 24 h in slow drying. This fi nding may clarify the increase in pyrochlorophyll pigment content. As shown in Fig. 4F, Phy-a had an upward trend in the drying process. This phenomenon may be infl uenced by the release of organic acid in the fruits after oil vacuole destruction; hence, the acidic environment activated the formation of Phy-a [26-27].
Chl degradation was initiated when fresh GPs were picked from the trees. A previous study demonstrated that the PAO pathway plays a crucial role in the decomposition of Chl pigments in GP-IOV. However, the Chl degradation pathways were intervened after oil vacuoles were damaged. Chl-a and Chl-b only constituted a very small part of the pigments in the fi nal dried GP-DOV (25 ℃), and the majority of pigments focused on Pheide-a (18%) and PyroPheide-a (27%), both of which presented a brown color.
Both Pheidase and Chlase represented a downward trend. However, the most signifi cant loss (72% of total Chl) was achieved within 24 h when GP-DOV was dried at 25 ℃, during which the two enzymes were detected at relatively high levels. The PAO degrading pathway seemed to be stagnant at the point of Pheide-a when oil vacuoles were destroyed. The accumulation of Pheide-a suggested a possible restriction in the conversion of Pheide-a to FCCs. In addition, the activated conversion of Pheide-a to PyroPheide-a also convinced this fact.
2.2.3 Changes in Chl-POD and associated Chl derivatives
As shown in Fig. 3D, the activity of Chl-POD in GP-DOV varied greatly in the different drying patterns. In fast drying, its activity was restrained and lowered gradually but showed a reverse “V” trend when dried at 25 ℃, peaking at 6.26 U/mg pro, which outweighed its highest activity (4.06 U/mg pro) in GP-IOV under the same drying condition. This phenomenon may be attributed to the adaptation of plants to the variations in environmental condition. Tissue damage or senescence can increase Chl-POD activity, which is very common in the post-harvest storage of fruits [18]. The following decrease in Chl-POD in slow drying may be attributed to fi erce dehydration. The signifi cant enhancement in Chl-POD activity during the 25 ℃ drying process also yielded complicated products that included different kinds of C13 2-OH-oxidized Chl pigments.
As shown in Fig. 4I, C13 2-OH Chl-a, the confirmed Chl catabolite of the peroxidase degrading pathway, also showed a reverse “V” trend in the two drying modes. This result disagreed with other findings that boosted Chl-POD activity could not prevent the continuous decrease of in vivo C13 2-OH Chl-a [10-13]. The initial increase in C13 2-OH Chl-a may be attributed to the strong Chl-POD activity and cell structural changes.
C13 2-OH Pheide-a (Fig. 4G), which was not detected in the fresh GPs, increased greatly in the two drying modes. C13 2-OH Chl-b (Fig.4H), which was absent in fast drying or GP-IOV drying, started to increase after 12 h of slow drying. C13 2-OH Phy-a (Fig. 4J), which was supposed to be converted from Phy-a, also showed an increasing trend in both drying modes [28]. The Chl-POD acquired from GP could also accept Pheide-a and Chl-b as substrates to generate C13 2-OH Pheide-a and C13 2-OH Chl-b, respectively. The oil vacuole damage helped to prompt these oxidizing reactions by increasing the accessibility of Chl-POD and Chls. This conclusion can be verifi ed by the sharp increase in C13 2-OH Chl catabolites in the beginning of GP-DOV slow drying. C15 1-OH-lactone Phy-a (Fig. 4K), which was not in the regular Chl degrading route, also increased in the 25 ℃ drying process. The formation of C15 1-OH-lactone Chl probably began with C13 2-OH-lactone Chl, which was also catalyzed by perioxidase [16]. Thus, the detected C13 2-OH Chl pigments with various forms resulted from the active catabolism of Chl-POD induced by the destruction of oil vacuoles.
GP underwent a rapid and sharp degradation of Chl when the oil vacuoles were destroyed. Fast drying appeared to be ineffective in retaining Chl. Chl catabolites that emerged in GP-DOV drying were more complicated and diverse than those in GP-IOV drying. The destruction of oil vacuoles facilitated several enzyme reactions but inhibited the reactions after the formation of Pheide-a in the PAO pathway. Therefore, the accumulation of Pheide-a and another branchconversion of Pheide-a to PyroPheide-a by Pheidase were highly induced in this procedure. PyroPheide-a accounted for the largest amount of Chl pigments in the dried GP-DOV. The higher Chl-POD activity was induced and accompanied by the accumulation of C13 2-OH-oxidized Chl in slow drying when oil vacuoles were damaged.
References:nces:
[1] CHEN Kewei, ZHANG Fusheng, KAN Jianquan. Characterization of chlorophyll breakdown in green prickleyashes (Zanthoxylum schinifolium Zucc.) during slow drying[J]. European Food Research and Technology, 2012, 234(6): 1023-1031.
[2] MATILE P, HÖRTENSTEINER S, THOMAS H. Chlorophyll degradation[J]. Annual Review of Plant Physiology and Plant Molecular Biology, 1999, 50: 67-95.
[3] SHIOI Y, TSUCHIYA T, TAKAMIYA K, et al. Conversion of chlorophyllide to pheophorbide by Mg-dechelating substance in extracts of Chenopodium album[J]. Plant Physiology and Biochemistry, 1996, 34(1): 41-47.
[4] SUZUKI T, SHIOI Y. Re-examination of Mg-dechelation reaction in the degradation of chlorophylls using chlorophyllin α as a substrate[J]. Photosynthesis Research, 2002, 74(2): 217-223.
[5] KUNIEDA T, AMANO T, SHIOI Y. Search for chlorophyll degradation enzyme, Mg-dechelatase, from extracts of Chenopodium album with native and artificial substrates[J]. Plant Science, 2005, 169(1): 177-183.
[6] HÖRTENSTEINER S. Chlorophyll degradation during senescence[J]. Annual Review of Plant Biology, 2006, 57: 55-77.
[7] BARRY C S. The stay-green revolution: recent progress in deciphering the mechanisms of chlorophyll degradation in higher plants[J]. Plant Science, 2009, 176(3): 325-333.
[8] KUNIEDA T, AMANO T, SHIOI Y. Characterization and cloning of the chlorophyll-degrading enzyme pheophorbidase from radish[J]. Plant Physiology, 2006, 140(2): 716-725.
[9] KUNIEDA T, AMANO T, SHIOI Y. Two enzymatic reaction pathways in the formation of pyropheophorbide a[J]. Photosynth Research, 2002, 74(2): 225-233.
[10] YAMAUCHI N, EGUCHI K. in vitro chlorophyll degradation involved in flavonoid radical formed by chlorophyll-degrading peroxidase in flavedo extract of citrus nagato-yuzukichi fruit[J]. Journal of the Japanese Society for Horticultural Science, 2002, 71(2): 243-248.
[11] CHENG M, MCPHEE K E, BAIK B K. Bleaching of green peas and changes in enzyme activities of seeds under simulated climatic conditions[J]. Journal of Food Science, 2004, 69(7): 511-518.
[12] COSTA M L, CIVELLO P M, CHAVES A R, et al. Effect of ethephon and 6-benzylaminopurine on chlorophyll degrading enzymes and a peroxidase-linked chlorophyll bleaching during post-harvest senescence of broccoli (Brassica oleracea L.) at 20 ℃[J]. Postharvest Biology and Technology, 2005, 35(2): 191-199.
[13] DISSANAYAKE P K, YAMAUCHI N, SHIGYO M. Chlorophyll degradation and resulting catabolite formation in stored Japanese bunching onion (Allium fistulosum L.)[J]. Journal of the Science of Food and Agriculture, 2008, 88(11): 1981-1986.
[14] FUNAMOTO Y, YAMAUCHI N, SHIGYO M. Involvement of peroxidase in chlorophyll degradation in stored broccoli (Brassica oleracea L.) and inhibition of the activity by heat treatment[J]. Postharvest Biology and Technology, 2003, 28(1): 39-46.
[15] ROCA M, GANDUL-ROJAS B, MÍNGUEZ-MOSQUERA M I. Varietal differences in catabolic intermediates of chlorophylls in Olea europaea (L.) fruit cvs. Arbequina and Blanqueta[J]. Postharvest Biology and Technology, 2007, 44(2): 150-156.
[16] VERGARA-DOMÍNGUEZ H, GANDUL-ROJAS B, ROCA M. Formation of oxidised chlorophyll catabolites in olives[J]. Journal of Food Composition and Analysis, 2011, 24(6): 851-857.
[17] FUKASAWA A, SUZUKI Y, TERAI H, et al. Effects of postharvest ethanol vapor treatment on activities and gene expression of chlorophyll catabolic enzymes in broccoli florets[J]. Postharvest Biology and Technology, 2010, 55(2): 97-102.
[18] AIAMLA-OR S, KAEWSUKSAENG S, SHIGYO M, et al. Impact of UV-B irradiation on chlorophyll degradation and chlorophylldegrading enzyme activities in stored broccoli (Brassica oleracea L. Italica Group) fl oret[J]. Food Chemistry, 2010, 120(3): 645-651.
[19] MÍNGUEZ-MOSQUERA M I, GALLARDO-GUERRERO L. Role of chlorophyllase in chlorophyll metabolism in olives cv. Gordal[J]. Phytochemistry, 1996, 41(3): 691-697.
[20] FRASER M S, FRANKL G. Detection of chlorophyll derivatives in soybean oil by HPLC[J]. Journal of the American Oil Chemists’Society, 1985, 62(1): 113-121.
[21] YANG Xiaotang, ZHANG Zhaoqi, JOYCE D, et al. Characterization of chlorophyll degradation in banana and plantain during ripening at high temperature[J]. Food Chemistry, 2009, 114(2): 383-390.
[22] YAMAUCHI N, AKIYAMA Y, KAKO S, et al. Chlorophyll degradation in Wase satsuma mandarin (Citrus unshiu Marc.) fruit with on-tree maturation and ethylene treatment[J]. Scientia Horticulturae, 1997, 71(1/2): 35-42.
[23] BRANDFORD M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding[J]. Analytical Biochemistry, 1976, 72(1/2): 248-254.
[24] ALMELA L, FERNANDEZ-LOPEZ J A, ROCA M J. Highperformance liquid chromatographic screening of chlorophyll derivatives produced during fruit storage[J]. Journal of Chromatography A, 2000, 870: 483-489.
[25] HUANG S C, HUNG C F, WU W B, et al. Determination of chlorophylls and their derivatives in Gynostemma pentaphyllum Makino by liquid chromatography-mass spectrometry[J]. Journal of Pharmaceutical and Biomedical Analysis, 2008, 48(1): 105-112.
[26] van BOEKEL M A J S. Testing of kinetic models: usefulness of the multiresponse approach as applied to chlorophyll degradation in foods[J]. Food Research International, 1999, 32(4): 261-269.
[27] KOCA N, KARADENIZ F, BURDURLU H S. Effect of pH on chlorophyll degradation and colour loss in blanched green peas[J]. Food Chemistry, 2006, 100(2): 609-615.
[28] HUFF A. Peroxidase catalyzed oxidation of chlorophyll by hydrogen peroxide[J]. Phytochemistry, 1982, 21(2): 261-265.
油胞破坏性青花椒干燥过程中叶绿素降解机制
阚建全
1,2,陈科伟
1,张甫生
1,2,郑 炯
1,2
(1.西南大学食品科学学院,重庆 400715;2.农业部农产品贮藏保鲜质量安全风险评估实验室(重庆),重庆 400715)
摘 要:青花椒在快速干燥过程中可以抑制叶绿素的降解,但油胞破坏性青花椒除外。为了探讨这个现象背后的发生机制,本实验将对油胞破坏性青花椒的叶绿素和相关酶的变化进行研究。结果表明,与油胞完整性青花椒相比,干燥后和油胞破坏性青花椒的叶绿素变化更加严重和复杂,干燥后的叶绿素仅剩下原来的6%。油胞破坏抑制了通过脱镁叶绿酸a氧化酶途径形成的焦脱植基叶绿素,并导致脱镁叶绿酸a的在青花椒体内积累(占总叶绿素的18%)。在慢速干燥过程中,逐渐增加的叶绿素降解过氧化物酶活力使得C13 2氧化型叶绿素的含量不断增加,相关的酶、叶绿素酶和脱镁叶绿素酸酶呈现出下降趋势,而叶绿素降解过氧化物酶和脱镁螯合物则出现波动变化。
关键词:青花椒;干燥;叶绿素降解
中图分类号:TS255.1
文献标志码:A
文章编号:1002-6630(2015)01-0019-08
收稿日期:2014-01-13
基金项目:国家自然科学基金面上项目(31071599)
作者简介:阚建全(1965—),男,教授,博士,研究方向为食品化学与营养学、食品安全与质量控制。E-mail:kanjianquan@163.com
doi:10.7506/spkx1002-6630-201501004