Isolation of Bacteriocinogenic Lactic Acid Bacteria for Biopreservation of Chinese Traditional Sichuan Pickle
RAO Yu
1, WANG Meng
1, JIANG Yunlu
1, CHANG Wei
1, CHEN Gong
2, XIANG Wenliang
1, CHE Zhenming
1,*
(1. Key Laboratory of Food Biotechnology of Sichuan Province, School of Bioengineering, Xihua University,Chengdu 610039, China; 2. Sichuan Academy of Food and Fermentation Industries, Chengdu 611130, China)
Abstract:Bacteriocinogenic lactic acid bacteria (LAB) were screened from eight Chinese traditional Sichuan pickle samples for Sichuan pickle biopreservation. A total of 8 strains isolated from the 227 presumptive LAB present in the pickle samples produced protease-sensitive bacteriocin-like substances (BLISs), which showed inhibitory activity against Listeria innocua,Staphylococcus aureus, Bacillus subtilis and Bacillus cereus. Among these 8 bacteriocinogenic strains, Lactobacillus harbinensis B22, Enterococcus faecium E6 and Lb. plantarum E11 produced BLISs against Gram-negative strain Escherichia coli. Biochemical analysis revealed that they were tolerant to acidity and high salt concentration. Meanwhile, BLISs B22, E6 and E11 remained stable under acidic condition and after heat treatment. In addition, all these three strainswere able to grow and produce BLISs in Sichuan pickle model medium, the cabbage juice medium containing 4 g/100 mL NaCl. It was clearly demonstrated that Lb. harbinensis B22, E. faecium E6 and Lb. plantarum E11 were desirable for biopreservation of Sichuan pickle.
Key words:bacteriocinogenic lactic acid bacteria; food biopreservation; screening; Sichuan pickle; cabbage juice medium
Biopreservation refers to preservation and extension of the shelf-life of food using microorganisms and/or their metabolits
[1]. Lactic acid bacteria (LAB) is considered to be generally recognized as safe (GRAS) and applied in food biopreservation, due to its competition to spoilage/pathogenic bacteria and its antimicrobial product of metabolism, such as organic acids, hydrogen peroxide, bacteriocins and antifungal peptides
[2]. The LAB bacteriocins are small,cationic, amphiphilic, ribosomally synthesized and vary in antimicrobial spectrum and action mode. During the past ten years, considerable attentions have been focused on the screening for new bacteriocins and bacteriocinogenic LAB with strong or wide antimicrobial activity. The bacteriocin preparations can be added as food additives for preservation
[3]. But the identification and purification of bacteriocins are difficult and costly, which restrict the practical application of bacteriocin. Above all, due to the legal restrictions, nisin is the sole bacteriocin permitted as biopreservative in food industries. The bacteriocinogenic LAB also can be inoculated as starter cultures or co-cultures to produce bacteriocin in situ for biopreservation. The in-situ production of bacteriocin in fermentation processes requires the bacteriocinogenic strain adapting to the particular food environment
[3-4]. Considering the acclimatization, LAB isolated from certain food products should be the best candidate as starter or co-cultures for the same food.
Pickles are made worldwide and have different styles,such as Sichuan Pickle in China, kimchi in Korea, sauerkraut and pickled cucumbers in the West. Sichuan pickle is one of the most popular traditional fermented pickles in China bec ause of its special flavor and taste. The production and sales of Sichuan pickle holds over half of pickle market in China. The Sichuan pickle is both homemade and factoryproduced and made of various fresh vegetables, such as cabbage, radish, bamboo shoot, tender ginger and chili. The vegetables are immersed into brine of 4-8 g/100 mL salt and spontaneous fermented with natural microorganisms present in raw materials
[5]. No sterilizing process makes the Sichuan pickle sensitive to deleterious bacteria, which were carried mainly by raw material. Pathogenic and spoilage bacteria, such as Listeria innocua, Staphylococcus aureus,Bacillus subtilis and Escherichia coli, could survive and grow during the fermentation process of pickles
[6-7]. Heat treatment and chemical preservatives could influence the organoleptic qualities and nutritional properties of pickles. Furthermore, chemical additives are of health threat to consumers. Biopreservation with bacteriocinogenic strain as starter culture or co-culture becomes the most nondestructive and safest preservation method for pickles
[8-9].
One of the crucial microorganisms in pickle fermentation is LAB, which accounts for 20%-30% of the total microbial amounts. Many researchers have studied the microflora from pickles of different countries and regions. The results showed that certain differences existed in microflora of various pickles
[10]. The Sichuan Basin, in the western hinterland of China, nearby the Qinghai-Tibet Plateau, has a specific humid sub-tropical monsoonal climate with mild winters, hot summers, plentiful rainfall, mist, high humidity and less sunshine. The special climate of Sichuan Basin and processing method made the microflora in Sichuan pickle different from other pickles. Lb. plantarum, Lb. brevis,and Lb. pentosus were the dominant LAB according to Yu Jie et al.
[5]. Tian Wei et al.
[11]reported that predominant species in Sichuan pickles are Lb. plantarum, Lb. paralimentarius and Pediococcus damnosus. In addition, Shang Jun et al.
[12]suggested that the microflora in pickles might be influenced by different raw materials. Due to the high microbial diversity, kinds of bacteriocinogenic LAB have already been selected from different pickles
[8,13]. But no bacteriocinogenic LAB were selected and used for biopreservation of Sichuan pickle so far.
In this study, several homemade and factory produced Sichuan pickles made of different vegetables were collected. The objective is to isolate bacteriocinogenic LAB with inhibitory activity against typical spoilage and pathogenic bacteria in food for Sichuan pickle biopreservation.
1 Materials and Methoddss
1.1 Sampling
Four factory-made and four homemade Sichuan pickles were collected in Sichuan Basin in western of China (Table 1). The raw materials of factory-produced pickles were cabbage,chili, radish and bamboo shoot respectively. Two homemade pickles were made of cabbage and tender ginger. Mixtures of vegetables were used in another two homemade pickles. The pickle juice samples were collected in the sterile eppendorf tubes, kept in an ice-box container, carried to the laboratory at 4 ℃.
1.2 LAB isolation and identification
Each pickle juice sample was diluted with sterile physiological saline (0.9 g/100 mL NaCl) to 10
-3-10
-4. The diluted sample was spread in de Man Rogosa Sharpe (MRS)agar supplemented with CaCO
3(5 g/L) and incubated at 30 ℃ for 1-2 days. Colonies with visible calcium-dissolved circles were picked out and spot on a new MRS agar. Grampositive and catalase negative strains were considered as presumptive LAB. 227 presumptive LAB from different pickle juice samples were cultured and stored in MRS with 15% glycerol at -70 ℃ (Table 1).
Table1 The LAB strains derived from different Sichuan pickle samples
Table1 The LAB strains derived from different Sichuan pickle samples

Note: 1. Factory-made Sichuan pickle were sampled from food comparies in Sichuan province in China; 2. Kinds of vegetables were used in Sichuan pickle.
SamplesRaw materialspHLAB numbersSampling point ACabbage3.824Factory-made
1BChili3.832Factory-made CRadish4.027Factory-made DBamboo shoot3.926Factory-made ECabbage3.429Homemade FTender ginger3.531Homemade GVegetables
23.727Homemade HVegetables3.531Homemade
After antibacterial assay, the bacteriocinogenic LAB isolates were subjected to genetic identification. Genomic DNA of each strain was extracted from overnight cultures using DNA extraction kits (Promega). The 16S rRNA gene sequences were amplified respectively by PCR. The specific PCR primers were the universal primer 27F (5’-AGAGTTTGATCCTGGCTCAG-3′;positions 8-27 of E. coli 16S rRNA) and primer 1490R (5’-GGTTACCTTGTTACGACTT-3’; positions 1491-1509 of E. coli 16S rRNA)
[14]. PCR was performed under the following conditions: pre-incubation at 95 ℃for 5 min, followed by 35 cycles of denaturation at 95 ℃for 1 min, annealing at 50 ℃ for 1 min and extension at 72 ℃ for 2 min, and a final extension at 72 ℃ for 5 min. The amplified 16S rRNA fragments were respectively cloned into a plasmid pMD19-T vector (TaKaRa) and subjected to DNA sequencing (HuaGene Biotech Co. Ltd., Shanghai, China). 1.3 Screening for bacteriocinogenic LAB
The presumptive lactic acid bacteria strains were cultured in MRS broth overnight at 30 ℃ for 24 h without agitation. Cell-free culture supernatants were collected by centrifugation at 8 000×g at 4 ℃ for 10 min. The supernatants were then adjusted to pH 6.0 with 1 mol/L NaOH to eliminate the interference of low pH value. Catalase(1 mg/mL, Sigma) was also added to supernatants to exclude the inhibition of hydrogen peroxide.
The antibacterial activity of the cell-free supernatants was determined by the agar well diffusion assay (AWDA)
[15]. Listeria innocua CICC 10416, Bacillus subtilis CICC 20551 and Escherichia coli CICC 21525 were used as an indicator strains for prescreening. 250 μL prepared cell-free neutralized supernatants were added into wells (8 mm in diameter) on solid media seeded with indicator strains. After incubation at 37 ℃ for 18 h, the diameters of inhibitory zones were determined. The neutralized supernatants with inhibitory activity were treated with pepsin (1 mg/mL, Amersco) to confirm the production of bacteriocin-like substances(BLISs). The presumptive BLISs were further tested against other indicator strains listed in Table 2. All the indicator strains were kept at -70 ℃ and were propagated in the appropriate culture media before use.
Table2 Antibacterial activity of LAB against the indicator strains
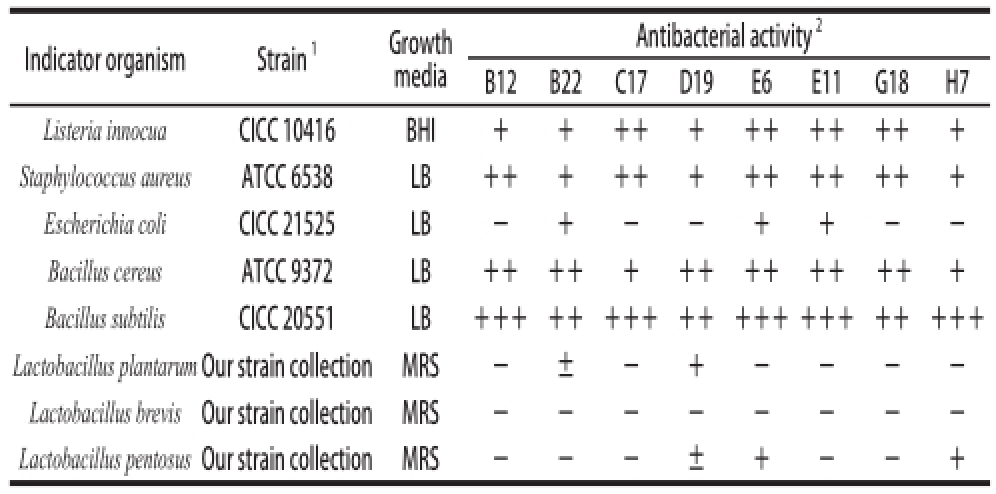
Note: 1. ATCC, American Type Culture Collection; CICC, China Center of Industrial Culture Collection; Our strain collections were selected in our lab from Sichuan pickles. 2. Antibacterial activity was defi ned as inhibition diameter (mm): +++. >18 mm; ++. 14-18 mm; +. 10-14 mm; ±. <10 mm; -. no inhibition.
Indicator organismStrain
1Growth media Antibacterial activity
2B12B22C17D19E6E11G18H7 Listeria innocuaCICC 10416BHI++++++++++++ Staphylococcus aureusATCC 6538LB+++++++++++++ Escherichia coliCICC 21525LB-+--++--Bacillus cereusATCC 9372LB+++++++++++ +++ Bacillus subtilisCICC 20551LB+++++++++++++++++++++ Lactobacillus plantarumOur strain collectionMRS-±-+----Lactobacillus brevisOur strain collectionMRS--------Lactobacillus pentosus Our strain collectionMRS---±+--+
1.4 Characterization of the antibacterial activity
L. innocua CICC 10416 was used as the indicator in this section. Proteinase K (Merck), pepsin (Amersco), papain(Sigma) and trypsin (Amersco) was used to further confirm the production of BLISs. The neutralized supernatants were supplemented with proteases above respectively at a final concentration of 0.5 mg/mL and incubated at 37 ℃ for 1 h. After enzyme denaturing by heating at 100 ℃ for 5 min,antibacterial activity of the supernatants was determined by AWDA described above. The control was the neutralized supernatants incubated without proteases.
The thermal stability of supernatants was detected. Neutralized supernatants were heated to 80, 100 and 121 ℃for 20 min. After each treatment, samples were cooled immediately and the antibacterial activity was tested.
The effect of pH on supernatants was tested. Neutralized supernatants were adjusted to pH values of 3.0, 4. 0, 5.0,6.0, 7.0, 8.0, 9.0, 10.0 and 11.0 by adding the appropriated volumes of 1 mol/L HCl or 1 mol/L NaOH. Antibacterial activity was determined immediately. Negative controls were sterile MRS broth with pH values of 3.0, 4.0, 5.0, 6.0, 7.0, 8.0,9.0, 10.0 and 11.0.
1.5 Morphological and physiological characteristics
Morphological properties of bacteriocinogenic LAB were examined by light microscopy. Gram staining and physiological tests, including motility, catalase test, nitratereduction, hydrolysis of gelatin, carbohydrates fermentation,production of acid were examined
[16]. The pH value of culture media was measured after 24 h incubation of LAB in MRS broth. The tolerance to NaCl was tested in MRS broth with different NaCl concentration (4-8 g/100 mL). Growth of LAB was measured spectrophotometrically after 24 h of incubation at 30 ℃. It was considered as positive if the optical density (OD) at 600 nm greater than 0.4.
1.6 Growth of LAB and BLISs production in MRS and cabbage juice medium
The Chinese cabbage were used to prepared cabbage juice with a juice extractor. Cabbage juice medium (CJM)with 4 g/100 mL NaCl, consisting of 60% cabbage juice and 40% water, was filtrated, autoclaved at 121 ℃ for 15 min and stored at 4 ℃ until use. 1 mL overnight bacteriocinogenic LAB strain culture was inoculated into 300 mL MRS and CJM respectively and incubated without agitation at 30 ℃for 36 h. 10 mL culture was sampled at 6 h intervals for test of antibacterial activity by AWDA. Meanwhile, the optical density (OD) at 600 nm and pH value was recorded. L. innocua CICC 10416 was used as the indicator in this section.
2 Results and Analysiiss
2.1 LAB Isolation and antibacterial assay
The list of eight traditional Sichuan pickle samples and their pH values was shown in Table 1. The pH values of the samples ranged from 3.5-4.0 and homemade pickles exhibited lower pH (3.4-3.7). 227 presumptive LAB were selected from these pickle samples, among which, 109 isolates from factory-made samples and 118 isolates from homemade samples.
The total 227 presumptive LAB were pre-screening for antibacterial activities against three typical spoilage and pathogenic bacteria by means of AWDA (Fig.1). The neutralized supernatants of 61 strains (26.9%) presented obvious inhibitory zones against B. subtilis. The neutralized supernatants of 17 strains (13.7%) showed inhibition against L. innocua and 7 strains (7.7%) showed inhibition against E. coli. Among these, 21 neutralized supernatants showed inhibition against two or three indicators. After treated with pepsin, only 8 neutralized supernatants remained antibacterial activities (Table 2). It suggested that the inhibitory active substances in the 8 supernatants were BLISs with proteinaceous nature.

Fig.1 Preliminary screening for antibacterial activity of LAB strains isolated from Sichuan pickles
The BLISs of the 8 LAB strains also showed inhibitory against Staphylococcus aureus and B. cereu, which were also spoilage and pathogenic bacteria in food product. Lb. plantarum,Lb. brevis and Lb. pentosus, which were the common strains in Sichuan pickles
[5], were used as indicators in further study. The BLISs of strain B12, C17, E11 and G18 showed no inhibition against these Sichuan pickle related strains. Lb. plantarum was influenced slightly by the BLISs of strain B22. Lb. pentosus was inhibited by the BLISs of strain D19, E6 and H7.
2.2 Strains identification
All the 8 strains shown in Table 2 were subjected to genetic identification by 16S rRNA sequencing. The result was showed in Fig.2. Strains B12, D19 and E6,which were respectively selected from factory-made chili,bamboo shoot pickle and homemade cabbage pickle, were E. faecium. Enterocins produced by enterococci are wellknown bacteriocins because of their diversity and wide inhibitory spectrum. Manybateriocinogenic enterococci were selected from food products and particularly inhibited against pathogenic bacteria such as L. monocytogenes, S. aureus and sometimes against Gram-negative bacteria like E. coli
[17-18]. In this study, E. faecium E6 could produce enterocin-likesubstance against both Gram-positive and Gram-negative bacteria. Enterocin genes, vancomycin resistance genotypes and potential enterococcal virulence factors of E. faecium B12, D19 and E6 were identified by PCR method described respectively by Vuyst
[19], Sylvie
[20]and Eaton
[21]et al.. E. faecium E6 had genes of enterocin P and L50B and no enterocin genes were detected in E. faecium B12 and D19(data not shown). Several results have been reported that one or more enterocin genes might occur in one enterococci strain and sometimes they couldn’t be detected because of the possible nucleotide substitutions
[22]. Absence of vancomycin resistance genotypes and potential enterococcal virulence factors in E. faecium B12, D19 and E6 suggested they were GRAS for biopreservation (data not shown).

Fig.2 Neighbor-Joining tree showing the phylogenetic position of eight LAB strains with inhibitory activities against typical spoilage and pathogen indicators and representatives of some other related taxa based on 16S rRNA sequences
Strains G18 and H7, which were selected from homemade pickles of mixed vegetables, were both Lb. rhamnosus. They produced BLISs only against Gram-positive pathogenic bacteria shown in Table 2. Several Lb. rhamnosus have been reported with production of bacteriocin against L. monocytogenes and some of them were selected from the pickles
[23].
Strain B22 selected from factory-made chili pickle,strain C17 selected from factory-made radish pickle,and strain E11 selected from homemade cabbage pickle,were respectively Lb. harbinensis, Lb. coryneformis and Lb. plantarum. Lb. coryneformis is always reported for its anti-fungal compounds, but seldom for the bacteriocin
[24]. The BLIS of Lb. coryneformis C17 in this study could inhibit against several Gram-positive strains and might be a new bacteriocin because of its different inhibitory spectrum.
2.3 Nature of BLISs from LAB
The BLISs of LAB strains B22, E6 and E11, which were inhibitory active against both Gram-positive and Gramnegative pathogenic bacteria, were further tested for protease,thermal and pH stability. As Table 3 shown, all of them were sensitive to protease except trypsin. The result further confirmed the production of BLISs.
Table3 Characterization of the antibacterial activity of LAB

Note: Antimicrobial activity was defi ned as inhibition diameter (mm): +++.>18 mm; ++. 14-18 mm; +. 10-14 mm; ±. <10 mm; -. no inhibition. The same as Table 4.
TreatmentLAB strains B22E6E11 Control+++++ Enzyme Proteinase K---Pepsin ---Papain ---Trypsin ±+± Heat-treatment 80 ℃ for 20 min++++ 100 ℃ for 20 min+++ 121 ℃ for 20 min++-
The BLISs of strains B22, E6 and E11 were resistant to heat-treatment at 80 ℃ for 20 min (Table 3). Compared with E. faecium E6 and Lb. plantarum E11, the BLIS of Lb. harbinensis B22 showed lower inhibitory activity (+: 10-14 mm inhibitory zone) against L. innocua but the strongest heat resistance. The BLIS of L. harbinensis B22 wasn’t influenced even after 121 ℃ treatment for 20 min. Compared with the control, the inhibitory activity of BLIS E6 decreased when treated at 100 ℃ and 121 ℃ for 20 min. The inhibitory activity of BLIS E11 was stable at 100 ℃ for 20 min but lost at 121 ℃ for 20 min.
The antibacterial activity of BLIS B22 could only be detected under acidic condition (Table 4). The antibacterial activity of BLIS E6 and E11 was recorded under acidic condition and decreased gradually with pH increase under alkaline condition. BLIS E6 lost the inhibitory activity at pH 10.0 and so was BLIS E11 at pH 11.0.
Table4 Effect of pH on the antibacterial activity of LAB

pHLAB strains ControlB22E6E11 3.0+++++++++ 4.0-++++++ 5.0-+++++ 6.0-+++++ 7.0-++++ 8.0--+++ 9.0--++ 10.0---± 11.0----
2.4 Morphological and biochemical characteristics of bacteriocinogenic LAB
The morphological and biochemical properties of Lb. harbinensis B22, E. faecium E6 and Lb. plantarum E11 were shown in Table 5. All these bacteriocinogenic LAB strains were able to ferment D-glucose, D-sucrose, D-lactose and D-fructose and produce lactic acid. E. faecium E6 and Lb. plantarum E11 were positive of motility and the former could reduce nitrate. After 24 h fermentation of the three LAB strains, the pH value of culture media could achieve 2.9-3.5. They could all grow in MRS containing 6 g/100 mL NaCl. E. faecium E6 and Lb. plantarum E11 were tolerant to 8 g/100 mL NaCl.
Table5 Phenotyptic characteristics of bacteriocinogenic LAB
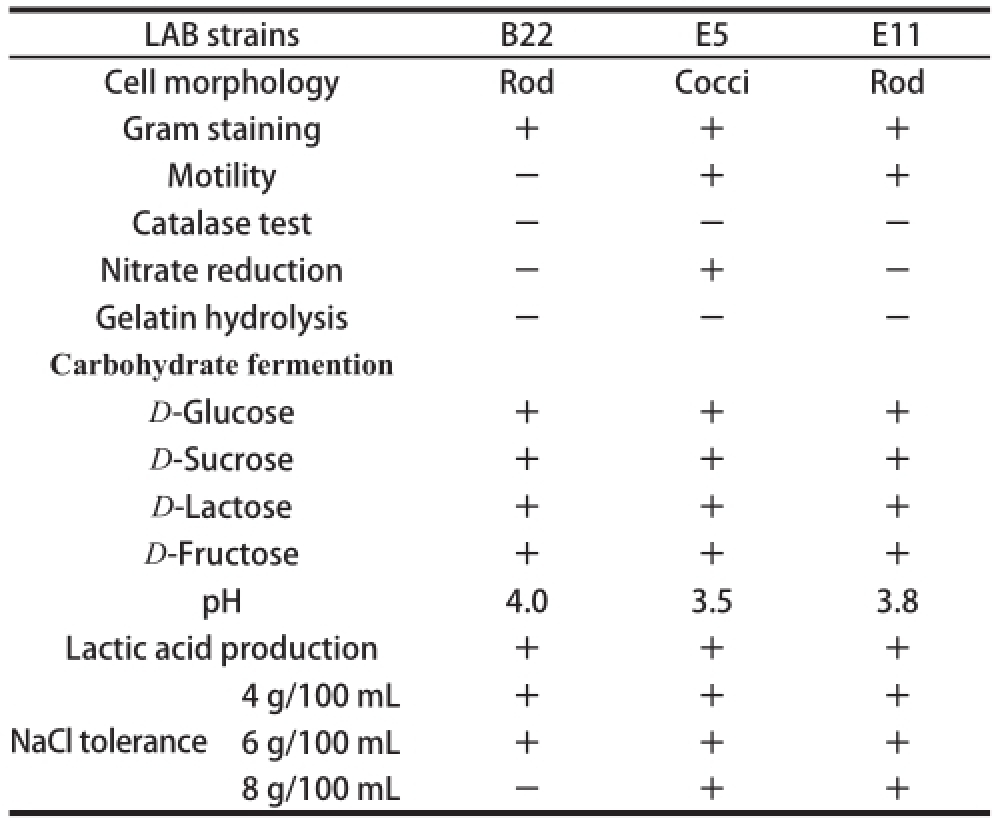
Note: The pH value of culture media after 24 h incubation of bacteriocinogenic LAB in MRS broth.
LAB strainsB22E5E11 Cell morphologyRodCocciRod Gram staining+++ Motility-++ Catalase test---Nitrate reduction-+-Gelatin hydrolysis---Carbohydrate fermention D-Glucose +++ D-Sucrose +++ D-Lactose +++ D-Fructose +++ pH4.03.53.8 Lactic acid production+++ 4 g/100 mL+++ 6 g/100 mL+++ 8 g/100 mL-++ NaCl tolerance
2.5 BLISs production in MRS and Sichuan pickle model medium
Food model systems are artificial media, which mimic the composition of natural food complex matrices for basicstudy of microorganisms
[10]. Chinese cabbage is a common vegetable used in Sichuan pickle. The CJM containing 4 g/100 mL NaCl was used to mimic the Sichuan pickle brine in this study. Compared with culturing in MRS broth, the growth and BLISs production of Lb. harbinensis B22, E. faecium E6 and Lb. plantarum E11 in CJM containing 4 g/100 mL NaCl were measured (Fig.3). Overall,the growth and BLIS production of Lb. harbinensis B22,E. faecium E6 and Lb. plantarum E11 were slower in CJB than in MRS broth at the beginning (0-12 h). For Lb. harbinensis B22, the growth and pH decline in CJM were obviously slower than in MRS. Lb. harbinensis B22 came to the logarithmic growth phase at 6 h and reach maximum optical density at 18 h in MRS, but later in CJM. The BLIS B22 was detected after 12 h fermentation in both MRS and CJM, but the production of BLIS B22 in MRS kept significantly higher than in CJM until 36 h. The difference of culture media and high NaCl concentration slightly influence the growth and BLIS production of E. faecium E6 and Lb. plantarum E11 at 24-36 h, especially the latter. In MRS broth, the acidification of E. faecium E6 and Lb. plantarum E11 was faster and the pH value was 0.3 higher than in CJB after 36 h incubation.

Fig.3 BLISs production during the growth of Lb. harbinenssiiss B22 (A),E. faecciiuumm E6 (B) anndd Lb. plantarruumm E11 (C) in MRS and CJM broth at 3300 ℃
3 Conclusiioonnss
Numerous bacteriocins and bacteriocinogenic LAB have been selected in recent years and their application appeared as a good alternative to chemical compounds and antibiotics
[10]. But each bacteriocin or bacteriocinogenic LAB has its special condition demands for antibacterial activity or growth and bacteriocin production, such as pH, temperature and carbohydrate sources. Therefore, one bacteriocin-based biopreservation could fit with a single food matrix and its application should be tested on a “product by product”basis
[25].
In this study, several Sichuan pickles were sampled and screened for bacteriocinogenic LAB for Sichuan pickle biopreservation. 8 bacteriocinogenic LAB were selected with wide inhibitory spectrum, among which, Lb. harbinensis B22, E. faecium E6 and Lb. plantarum E11 could produce BLISs against both Gram-positive and Gramnegative strain. Their growth and BLIS products had a little or little influence with Sichuan pickle fermentation-related LAB. They could be tolerant to high NaCl concentration(4-8 g/100 mL), which is also the salt condition of Sichuan pickle. The pH range of Sichuan pickle is usually 3.5-4.0, under which, the BLISs of Lb. harbinensis B22, E. faecium E6 and Lb. plantarum E11 remained antibacterial activities. The BLISs of Lb. harbinensis B22, E. faecium E6 and Lb. plantarum E11 showed satisfactory thermal stability,represented antibacterial activity after heating treatment at 100 ℃ for 20 min. Most importantly, Lb. harbinensis B22,E. faecium E6 and Lb. plantarum E11 could growth and produce BLISs in CJM containing 4 g/100 mL NaCl, which was the model medium of Sichuan pickle. The result suggested the ability of BLIS production in situ of Lb. harbinensis B22,E. faecium E6 and Lb. plantarum E11 in Sichuan pickle.
In conclusion, this study strongly suggested that Lb. harbinensis B22, E. faecium E6 and Lb. plantarum E11 had desirable characteristics and were valuable for Sichuan pickle biopreservation. The practice application of these bacteriocinogenic LAB strains as starter culture or co-culture in Sichuan pickle production will be further investigated.
References:nces:
[1] ROSS R P, MORGANA S, HILLB C. Preservation and fermentation: past, present and future[J]. International Journal of Food Microbiology,2002, 79(1/2): 3-16.
[2] REIS J A, PAULA A T, CASAROTTI S N, et al. Lactic acid bacteria antimicrobial compounds: characteristics and applications[J]. Food Engineering Reviews, 2012, 4(2): 124-140.
[3] de VUYST L, LEROY F. Bacteriocins from lactic acid bacteria: production, purification, and food applications[J]. Journal of Molecular Microbiology Biotechnology, 2007, 13(4): 194-199.
[4] TRIAS R, BADOSA E, MONTESINOS E, et al. Bioprotective Leuconostoc strains against Listeria monocytogenes in fresh fruits and vegetables[J]. International Journal of Food Microbiology, 2008,127(1/2): 91-98.
[5] YU Jie, GAO Wa, QING Manjun, et al. Identification and characterization of lactic acid bacteria isolated from traditional pickles in Sichuan, China[J]. Jounal of General and Applied Microbiology,2012, 58(3): 163-172.
[6] BREDT J F, CALDWELL J M. Survival of Escherichia coli O157:H7 in cucumber fermentation brines[J]. Journal of Food Science, 2011,76(3): M198-M203.
[7] MAKLON K, MINAMI A, KUSUMOTO A, et al. Isolation and characterization of Listeria monocytogenes from commercial asazuke(Japanese light pickles)[J]. International Journal of Food Microbiology,2010, 139(3): 134-139.
[8] CHANG J Y, CHANG H C. Growth inhibition of foodborne pathogens by kimchi prepared with bacteriocin-producing starter culture[J]. Journal of Food Science, 2011, 76(1): M72-M78.
[9] SETTANNI L, CORSETTI A. Application of bacteriocins in vegetable food biopreservation[J]. International Journal of Food Microbiology,2008, 121(2): 123-138.
[10] XIONG Tao, GUAN Qianqian, SONG Suhua, et al. Dynamic changes of lactic acid bacteria flora during Chinese sauerkraut fermentation[J]. Food Control, 2012, 26(1): 178-181.
[11] TIAN Wei, ZHANG Qing, DENG Zhenzhen, et al. The bacterial diversity in Chinese traditionally fermented Sichuan pickle as investigated by 16S rRNA sequence analysis[J]. Food Science, 2013,34(17): 215-218.
[12] SHANG Jun, ZHONG Fangxu, SUN Yi, et al. Separation and screening of lactic acid bacteria form several traditional fermented vegetables[J]. Food Science, 2007, 28(4): 195-199.
[13] GAO Yurong, JIA Shiru, GAO Qiang, et al. A novel bacteriocin with a broad inhibitory spectrum produced by Lactobacillus sake C2, isolated from traditional Chinese fermented cabbage[J]. Food Control, 2010,21(1): 76-81.
[14] XIANG Wenliang, GUO Jianhua, FENG Wei, et al. Community of extremely halophilic bacteria in historic Dagong Brine Well in southwestern China[J]. World Journal of Microbiology and Biotechnology, 2008, 24: 2297-2305.
[15] SCHILLINGER U, LUCKE F K. Identification of lactobacilli from meat and meat products[J]. Food Microbiology, 1987, 4(3): 199-208.
[16] SMIBERT R M, KREIG N R. Phenotypic characteriztion[M]// GERHARDT P, MURRAY R G E, COSTILOW R N, et al. Manual of m ethods for general bacteriology. Washington: American Society for Microbiology, 1994.
[17] BELGACEM Z B, ABRIOUEL H, OMAR N B, et al. Antimicrobial activity, safety aspects, and some technological properties of bacteriocinogenic Enterococcus faecium from artisanal Tunisian fermented meat[J]. Food Control, 2010, 21(4): 462-470.
[18] THEPPANGNA W, MURASE T, TOKUMARU N, et al. Screening of the enterocin genes and antimicrobial activity against pathogenic bacteria in Enterococcus strains obtained from different origins[J]. Journal of Veterinary Medical Science, 2007, 69(12): 1235-1239.
[19] VUYST L D, FOULQUIE MORENOA M R, REVETSB H. Screening for enterocins and detection of hemolysin and vancomycin resistance in enterococci of different origins[J]. International Journal of Food Microbiology, 2003, 84(3): 299-318.
[20] SYLVIE D M, STEFAN E, PATRICE C. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR[J]. Journal of Clinic Microbiology, 1995,33(1): 24-27.
[21] EATON T J, GASSON M J. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates[J]. Applied and Environmental Microbiology, 2001, 67(4): 1628-1635.
[22] STROMPFOVA V, LAUKOVA A, SIMONOVA M, et al. Occurrence of the structural enterocin A, P, B, L50B genes in enterococci of different origin[J]. Veterinary Microbiology, 2008, 132(3/4): 293-301.
[23] TODOROV S D, FURTADO D N, SAAD S M I, et al. Potential beneficial properties of bacteriocin-producing lactic acid bacteria isolated from smoked salmon[J]. Journal of Applied Microbiology,2011, 110(4): 971-986.
[24] MAGNUSSON J, STROM K, ROOS S, et al. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria[J]. FEMS Microbi ology Letters, 2003, 219(1): 129-135.
[25] COSENTINO S, FADDA M E, DEPLANO M, et al. Antilisterial activity of Nisin-like bacteriocin-producing Lactococcus lactis subsp. lactis isolated from traditional sardinian dairy products[J]. Journal of Biomedicine and Biotechnology, 2012. doi: 10.1155/2012/376428.
筛选用于四川泡菜生物保鲜的产细菌素乳酸菌
饶 瑜
1,王 猛
1,蒋云璐
1,常 伟
1,陈 功
2,向文良
1,车振明
1,*
(1.西华大学生物工程学院,食品生物技术四川省高校重点实验室,四川 成都 610039;2.四川省食品发酵工业研究设计院,四川 成都 611130)
摘 要:从8 种中国传统四川泡菜中筛选产细菌素的乳酸菌,以用于四川泡菜的生物保鲜。从四川泡菜样品中共分离出227 株乳酸菌,并筛选出8 株能够产生细菌素类化合物抑制英诺克李斯特氏菌(Listeria innocua)、金黄色葡萄球菌(Staphylococcus aureus)、枯草芽孢杆菌(Bacillus subtilis)和蜡样芽孢杆菌(Bacillus cereus)生长的乳酸菌。其中Lactobacillus harbinensis B22、屎肠球菌E6(Enterococcus faecium E6)和植物乳杆菌E11(Lb. plantarum E11)能够产生抑制革兰氏阴性菌大肠杆菌(Escherichia coli)生长的细菌素类化合物。进一步研究表明,这3 株乳酸菌耐酸、耐高盐,能够在四川泡菜模拟培养基中生长并产生耐酸耐高温的细菌素类化合物。结果表明,Lactobacillus harbinensis B22、屎肠球菌E6和植物乳杆菌E11可用于四川泡菜的生物保鲜。
关键词:产细菌素乳酸菌;食品生物保鲜;筛选;四川泡菜;卷心菜汁培养基
中图分类号:TS201.3
文献标志码:A
文章编号:1002-6630(2015)03-0171-07
doi:10.7506/spkx1002-6630-201503033
收稿日期:2014-02-21
基金项目:四川省教育厅重点项目( 13ZA0026);西华大学重点科研基金项目(Z1120536);西华大学重点实验室开放研究基金项目(szjj2013-047)
作者简介:饶瑜(1982—),女,副教授,博士,研究方向为食品微生物。E-mail:ryfish@163.com
*通信作者:车振明(1960—),男,教授,学士,研究方向为食品微生物。E-mail:czm@mail.xhu.edu.cn







