
图1 尿石素A、B和C的化学结构
Fig.1 Chemical structures of UroA, UroB and UroC
尿石素A. R1—H,R2—OH,R3—OH;尿石素B. R1—H,R2—H,R3—OH;尿石素C. R1—OH,R2—OH,R3—OH。
尹培培1,闫林林1,曹若愚1,陈晓媛2,马 超1,*,刘玉军1,*
(1.北京林业大学生物科学与技术学院,北京 100083;2.山东省食品药品检验研究院,山东 济南 250101)
摘 要:鞣花单宁和鞣花酸是广泛分布于多种蔬菜、水果和坚果中的多酚类抗氧化剂,其在胃肠道的吸收较差,生物利用率较低,但部分会被哺乳动物肠道微生物转化为更易吸收的尿石素类代谢产物。因此,人们推测这些代谢产物可能为鞣花单宁及鞣花酸发挥生物学活性的最终物质基础。本文对近年来国内外有关尿石素化学性质和抗氧化、抗炎、抗癌以及调节肠道菌群等生物活性的研究进行综述,以期为富含鞣花单宁及鞣花酸类食品的科学利用提供借鉴。
关键词:尿石素;鞣花酸;抗氧化;抗炎;抗癌
鞣花酸是广泛分布于石榴、草莓、黑莓及胡桃等众多水果或坚果中的天然多酚类抗氧化剂,其在自然界中主要以缩合形式——鞣花单宁存在。人体及动物实验表明,鞣花单宁和鞣花酸均具有抗氧化、抗炎、抗癌及调节肠道菌群等多种生物活性,对癌症、糖尿病、心脑血管疾病和神经性病变等慢性疾病具有潜在的预防或治疗功效[1]。然而,鞣花单宁和鞣花酸的生物利用度极低,最终分布于组织及血液中的鞣花酸浓度往往低于其发挥生物学功效的有效浓度,部分未被吸收的鞣花酸则在哺乳动物胃肠道菌群的作用下代谢成更易吸收的尿石素(urolithin)类物质[2-6]。因此,有报道认为尿石素类物质可能是鞣花单宁和鞣花酸在体内发挥生物活性的物质基础[3,5,7]。近年来,有关尿石素生物活性、体内代谢过程与组织分布等相关的研究引起了人们的广泛关注。
尿石素A(UroA)和尿石素B(UroB)最早是从羊肾结石中分离得到的鞣花酸代谢产物[8]。天然尿石素在自然界中并不常见,但作为鞣花单宁或鞣花酸的代谢产物却广泛分布于人、大鼠、小鼠、牛、猪等哺乳动物的尿液、粪便和胆汁中[9-11]。Espín等[12]研究发现,尿石素可在伊比利亚猪的膀胱和胆囊中富集至较高浓度,而在肌肉、脂肪、肾脏、肝脏、心脏等其他组织中则无明显富集现象。小鼠[13]及人体实验[14-15]研究发现,尿石素及其衍生物在前列腺组织中有富集现象,在人结肠组织中也有分布[16]。
尿石素是由鞣花酸失去一个内酯环,并逐步脱羟基而生成的[17]。鞣花酸失掉内酯环后首先得到尿石素M-5(UroM-5),UroM-5不同位置脱羟基后生成尿石素D (UroD)、尿石素M-6(UroM-6)等几种四羟基尿石素异构体,四羟基尿石素失掉一个羟基后得到尿石素C (UroC)、尿石素M-7(UroM-7)等三羟基尿石素,三羟基尿石素再失掉一个羟基得到UroA和尿石素A异构体(isoUroA)等二羟基尿石素,最后得到单羟基的尿石素B (UroB),且isoUroA较UroA更易脱羟基生成UroB[9]。García-Villalba等[18]采用肠道微生物体外代谢实验发现了鞣花酸的代谢物UroM-5、UroM-6、UroM-7、UroC和尿石素E(UroE),首次证实尿石素是在肠道菌群的作用下生成的。
尿石素的特征性紫外图谱可用来鉴别具有不同羟基取代的尿石素及其甲酯、葡糖醛酸酯或硫酸酯等衍生物[18]。尿石素在甲醇中的紫外图谱显示其在240~400 nm处有两个主要的吸收峰,分别是峰1:300~380 nm和峰2:240~280 nm,许多情况下,也能检测到280~300 nm处的峰。峰1和峰2与尿石素的特征环并没有关系[19],但借助于紫外图谱可区分出C-9位置是否有羟基取代,如UroA的异构体在9号位置上有羟基,其峰1蓝移,峰2的吸收有增加,最大吸收波长在256 nm处,而256 nm也是C-9位有羟基取代的尿石素类物质主要吸收峰[9]。尿石素A、B、C的化学结构见图1。

图1 尿石素A、B和C的化学结构
Fig.1 Chemical structures of UroA, UroB and UroC
尿石素A. R1—H,R2—OH,R3—OH;尿石素B. R1—H,R2—H,R3—OH;尿石素C. R1—OH,R2—OH,R3—OH。
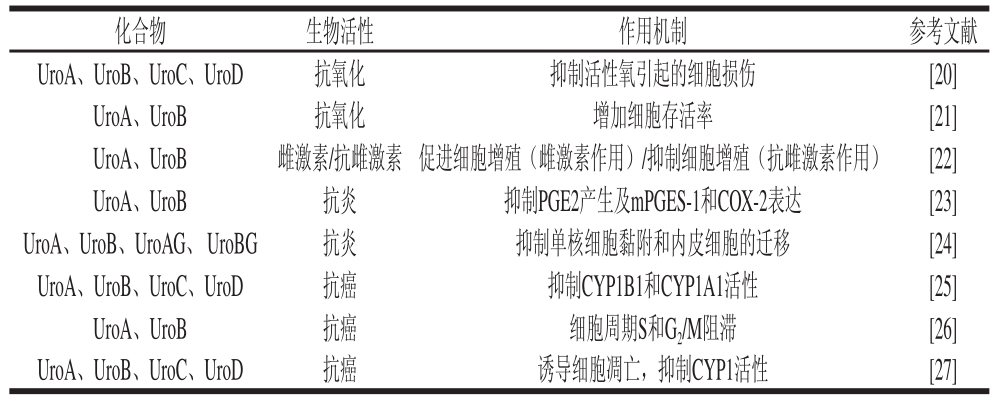
近年来,有关尿石素生物活性的研究较多,其中以抗氧化、抗炎、抗癌、调节肠道菌群、类雌激素/抗雌激素作用以及蛋白糖基化抑制作用等生理活性的研究较为集中(表1)。
表1 尿石素对人类细胞系的生物活性
Table 1 Biological activities of urolithins in human cell lines
2.1 抗氧化活性
鉴于尿石素的前体物质鞣花单宁及鞣花酸具有显著的抗氧化活性,有关尿石素抗氧化活性的研究报道较多。氧自由基吸收能力(oxygen radical absorbance capacity,ORAC)实验表明,尿石素类代谢产物均具有一定的抗氧化活性,其中UroA的抗氧化活性最强[28],仅次于原花色素寡聚物、儿茶素、表儿茶素和3,4-二羟基苯乙酸等[29]。然而,与鞣花单宁及鞣花酸相比,尿石素的抗氧化活性显著降低,如安石榴苷的1,1-二苯基-2-三硝基苯肼(1,1-diphenyl-2-picrylhydrazyl,DPPH)自由基清除活性是UroA的42 倍,2,2’-联氮-二(3-乙基-苯并噻唑-6-磺酸)二铵盐(2,2’-azinobis-(3-ethylbenzthiazoline-6-sulphonate),ABTS)自由基清除活性是其3 500 倍[3]。在其他几个抗氧化实验中,尿石素也表现出一定的抗氧化活性,其IC50值均在100 μmol/L以上[28]。但由于其细胞跨膜转运效率较高,部分尿石素在体内抗氧化实验中表现出较高的活性,如Bialonska等[20]发现,在细胞抗氧化实验中,UroC和UroA 的IC50值分别为0.16 μmol/L和13.6 μmol/L,而在同一实验中鞣花酸和VC 的IC50值分别为1.1 μmol/L和1.9 μmol/L。Haddad等[30]发现,人食用胡桃后,尿液中UroA的水平明显升高,餐后生育酚、儿茶酚水平显著升高,氧化应激反应显著降低。通过氧化应激诱导神经细胞发现,UroB (0.5~20 µmol/L)和UroA(10 µmol/L)可增加细胞的存活率,表现出显著的细胞保护作用[21]。UroA、UroB、8-甲氧基尿石素(8-OMe-Uro)及鞣花酸可显著降低H2O2诱导的人膀胱癌T24细胞中活性氧(reactive oxygen species,ROS)及丙二醛(malondialdehyde,MDA)的水平,并能提高超氧化物歧化酶(superoxide dismutase,SOD)的活性[31]。近来研究发现尿石素不仅有抗氧化作用,也有助氧化作用。Kallio等[32]发现,尿石素在ORAC实验中抗氧化能力比较强,而在细胞及Copper-initiated Prooxidant Activity(CIPA)实验中具有促氧化作用。
2.2 抗炎活性
结肠成纤维细胞在肠道免疫反应中发挥着重要作用,尿石素能显著抑制白细胞介素-1b(interleukin-1b,IL-1b)或肿瘤坏死因子-a(tumor necrosis factors-a,TNF-a)诱导的结肠成纤维细胞的炎症反应,降低炎性因子前列腺素E2(prostag landin E2,PGE2)的表达水平,其中UroA活性最强,UroB次之,鞣花酸活性最弱。UroA还能显著抑制IL-1b或TNF-a诱导的结肠成纤维细胞核转录因子(nuclear transcription factor-κB,NF-κB)与丝裂原活化蛋白激酶(mitogen-activated protein kinases,MAPK)的活化,下调环氧合酶-2(cyclo-oxygenase-2,COX-2)和膜结合型前列腺素E2合酶-1(microsomal PGE synthase-1,mPGES-1)等PGE2合成关键酶的表达量,进而减少PGE2的水平[23]。UroB与UroC可显著抑制组蛋白乙酰转移酶(histone acetylase,HAT)活性,而组蛋白的乙酰化或去乙酰化与炎症相关转录因子NF-κB 和AP-1的激活与失活相关,因此UroB与UroC在炎症中也发挥重要作用[33]。尿石素的葡糖醛酸衍生物也具有一定的抗炎活性,如UroA与UroB的葡萄糖醛酸酯能显著抑制TNF-a,诱导人主动脉内皮细胞的迁移及单核细胞的黏附,显著降低CC趋化因子配体2(chemokine(C-C motif)ligand 2,CCL2)和纤溶酶原激活物抑制物-1 (plasminogen activator inhibitor 1,PAI-1)的表达,其活性与UroA、UroB的活性相当[24],提示UroA与UroB的葡糖醛酸酯衍生物可能是石榴汁抑制心血管系统炎症反应的重要活性物质[34]。
2.3 抗癌活性
体外研究表明,尿石素能有效抑制前列腺癌、结肠癌及膀胱癌细胞的增殖。尿石素在体内的分布具有显著的组织特异性,如食用石榴汁或胡桃后,代谢产物尿石素可富集于人的前列腺组织中,因此有望用于前列腺相关疾病的治疗[20]。Vicinanza等[35]发现,石榴汁代谢物鞣花酸及UroA能抑制雄激素依赖的前列腺癌细胞的增殖,且具有协同抑制作用。Stolarczyk等[36]发现,UroC能显著抑制前列腺细胞系LNCaP的增殖(IC50=35.2 μmol/L),降低前列腺特异性抗原(prostatespecific antigen,PSA)的表达量,显著抑制精氨酸酶的活性,因此UroC可能为柳叶菜属(Epilobium)植物预防或治疗前列腺癌的重要活性物质。人细胞色素P450氧化酶CYP1B1是前列腺癌化疗的重要靶点,CYP1B1抑制剂在肿瘤的发生、发展及耐药性形成过程中均发挥着重要作用。Kasimsetty 等[25]通过体外重组CYP1B1介导的7-乙氧基异吩恶唑脱乙基酶(ethoxyresorufin-O-deethylase,EROD)活性分析发现,尿石素可以通过抑制CYP1B1的活性并降低CYP1B1蛋白的表达量对其进行双重调节。其中,UroA与UroB 是CYP1B1的特异性抑制剂,其选择性是CYP1A1的2~3倍,其前体物质Punicalins与Punicalagins则是CYP1A1的特异性抑制剂,其选择性是CYP1B1的5~10倍。鞣花酸及其代谢产物尿石素对人结肠癌Caco-2细胞的增殖也具有显著的抑制作用,其机制主要与细胞S和G2/M周期阻滞,成纤维和表皮生长因子受体(fibroblast growth factor 2/epidermal growth factor receptor,FGFR2/EGFR),癌基因K-Ras、c-Myc,肿瘤抑制因子DUSP6、Fos以及细胞周期相关基因CCNB1、CCNB1IP1等的表达调控相关[26]。Kasimsetty等[27]发现,UroA、UroB、UroC和UroD在浓度为25~50 µmol/L时即可诱导人结肠癌HT-29细胞发生凋亡,在50~75 µmol/L时能抑制50%的人细胞色素P450氧化酶CYP1的活性,而500 µmol/L时能诱导细胞周期阻滞。Qiu Zhenpeng等[31]发现,UroA、UroB、8-OMe-Uro及鞣花酸在体外可显著抑制膀胱癌T24细胞系的增殖,其IC50分别为43.9、35.2、46.3、33.7 µmol/L,其抑制作用与p38-MAPK通路和/或c-Jun介导的Caspase-3激活以及T24细胞内氧化应激状态的降低相关。
尿石素类代谢产物可以抑制蛋白激酶CK2及拓扑异构酶Ⅱ等与肿瘤相关酶的活性,并可影响肿瘤细胞的耐药性。Cozza等[37]发现,鞣花酸和UroA在体外可显著抑制蛋白激酶CK2酶的活性,其中UroA活性较强,其IC50为0.39 µmol/L。鞣花酸及尿石素类物质还是人拓扑异构酶Ⅱα与β型同工酶的竞争性抑制剂,在低于1 µmol/L的浓度水平即可与ATP竞争拓扑异构酶中ATP结合位点,并表现出显著的量效关系[38]。乳腺癌耐药蛋白(breast cancer resistance protein,BCRP/ABCG2)是介导肿瘤细胞耐药的一种重要蛋白,严重影响癌症的化疗效果。Gonzalez-Sarrias等[39]发现,UroA及其硫酸酯衍生物可作为BCRP/ABCG2的底物,可以剂量依赖性地抑制抗肿瘤药物米托蒽醌的转运。这说明鞣花单宁及鞣花酸的肠道代谢物能够调节ABCG2/BCRP介导的细胞转运过程和癌症耐药机制。
2.4 调节肠道菌群
肠道菌群通过群体感应(quorum sensing,QS)产生、释放一些特定的信号分子进行信息交流,进而调节微生物的群体行为和基因表达。哺乳动物肠道病原体小肠结肠炎耶尔森菌(Yersinia enterocolitica)能产生N-己酰高丝氨酸内酯(C6-HSL)与N-(3-氧桥)-己酰高丝氨酸内酯(3-oxo-C6-HSL)两种重要的N-酰基高丝氨酸内酯(N-acyl-homoserine lactones,AHLs)类信号分子,这些AHLs信号分子在QS介导的肠道感染过程中发挥着重要作用。研究发现[40],UroA与UroB能显著降低Y. enterocolitica中C6-HSL与3-oxo-C6-HSL信号分子的表达量,抑制QS相关的生物膜形成与运动过程,但这种抑制作用与AHLs合成相关基因yenI和yenR,以及运动相关基因flhDC、fliA和fleB的表达下调无关。由此可见,尿石素类化合物可以通过肠道菌群的群体感应抑制致病菌的生长,从而维持肠道菌群的平衡。
2.5 雌激素受体调节剂
植物源类雌激素/抗雌激素物质具有调节胆固醇水平、维持绝经后骨密度等多种功效。研究发现,UroA与UroB的特殊分子结构使其容易与α-和β-雌激素受体结合[22],但由于其取代基不同,其类雌激素作用也存在差异[41],其中UroA对雌激素α-和β-受体的亲和性比UroB大,并且UroA更容易结合雌激素α-受体。UroA与UroB可剂量依赖性促进对雌激素敏感的人乳腺癌细胞系MCF-7的增殖,呈现出弱雌激素样作用,且浓度高达40 μmol/L时,也不会抑制MCF-7细胞的增殖或表现出细胞毒性作用。尿石素还具有一定的抗雌激素作用,可剂量依赖性抑制雌二醇诱导的MCF-7细胞的增殖作用[22]。
2.6 抑制蛋白糖基化
蛋白质糖基化终产物是高血糖症的次生效应,其与心脑血管疾病、糖尿病及老年痴呆等疾病密切相关[42]。研究发现,UroA和UroB(1 µmol/L)可显著抑制蛋白质的糖基化,其中UroA呈显著的量效关系,而UroB则不存在明显的量效关系。尿石素的这种蛋白质糖基化抑制作用与其抗氧化活性及乙二醛结合能力无关[21]。
目前,国际上对鞣花酸及富含鞣花单宁与鞣花酸的食物在不同动物体内代谢生成尿石素的种类及含量的变化规律已有较多报道,随着高效液相色谱-串联质谱联用(high performance liquid chromatography-mass spectrometry/mass spectrometry,HPLC-MS/MS)等高通量、高灵敏度检测方法的应用,更多的尿石素及其衍生物陆续被发现,有关尿石素生物学活性的研究也逐渐成为新的研究热点,这对系统评价肠道菌群的代谢过程对鞣花单宁及鞣花酸生物活性的影响提供了更多的理论支撑。但是,目前关于尿石素的生物活性研究多为基于酶活性或细胞模型的体外活性评价,整体动物实验和人体实验数据十分有限,仅有极少数文献报道了尿石素的体内抗氧化[43-44]及其潜在化疗作用[43]。因此,有必要对尿石素开展更多整体动物实验或人体实验,并结合营养代谢组学等方法全面分析肠道菌群代谢过程及尿石素种类和含量的变化对鞣花单宁及鞣花酸生物活性的影响。鉴于我国关于尿石素的研究刚刚起步,更需要进一步开展尿石素构效关系、体内代谢与分布规律、安全性评价及我国人群肠道菌群结构对其代谢规律等方面的研究,这对于富含鞣花酸类食品的科学合理利用具有重要意义。
参考文献:
[1]SEERAM N P, SCHULMAN R N, HEBER D. Pomegranates: ancient roots to modern medicine[M]. Boca Raton: CRC Press, 2006: 244.
[2]CERDÁ B, LLORACH R, CERÓN J J, et al. Evaluation of the bioavailability and metabolism in the rat of punicalagin, and antioxidant polyphenol of pomegranate juice[J]. European Journal of Nutrition, 2003, 42(1): 18-28.
[3]CERDÁ B, ESPÍN J C, PARRA S, et al. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolized into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora in healthy humans[J]. European Journal of Nutrition, 2004, 43(4): 205-220.
[4]CERDÁ B, TOMÁS-BARBERÁN F A, ESPÍN J C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts and oak-aged wine in humans: identification of biomarkers and individual variability[J]. Journal of Agricultural and Food Chemistry, 2005, 53(2): 227-235.
[5]SEERAM N P, HENNING S M, ZHANG Y J, et al. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours[J]. Journal of Nutrition, 2006, 136(10): 2481-2485.
[6]MERTENS-TALCOTT S U, JILMA-STOHLAWETZ P, RIOS J, et al. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy human volunteers[J]. Journal of Agricultural and Food Chemistry, 2006, 54(23): 8956-8961.
[7]LARROSA M, GARCÍA-CONESA M T, ESPÍN J C, et al. Ellagitannins, ellagic acid and vascular health[J]. Molecular Aspects of Medicine, 2010, 31(6): 513-539.
[8]LEDERER E. Chemistry and biochemistry of some mammalian secretions and excretions[J]. Journal of the Chemical Society, 1949, 2115-2125.
[9]ESPÍN J C, LARROSA M, GARCÍA-CONESA M T, et al. Biological significance of urolithins, the gut microbial ellagic acid derived metabolites: the evidence so far[J]. Evidence-Based Complementary and Alternative Medicine, 2013, doi: 10.1155/2013/270418.
[10]TULIPANI S, URPI-SARDA M, GARCIA-VILLALBA R, et al. Urolithins are the main urinary microbial-derived phenolic metabolites discriminating a moderate consumption of nuts in free-living subjects with diagnosed metabolic syndrome[J]. Journal of Agricultural and Food Chemistry, 2012, 60(36): 8930-8940.
[11]GONZALEZ-BARRIO R, TRUCHADO P, GARCIA-VILLALBA R, et al. Metabolism of oak leaf ellagitannins and urolithin production in beef cattle[J]. Journal of Agricultural and Food Chemistry, 2012, 60(12): 3068-3077.
[12]ESPÍN J C, GONZÁLEZ-BARRIO R, CERDÁ B, et al. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans[J]. Journal of Agricultural and Food Chemistry, 2007, 55(25): 10476-10485.
[13]SEERAM N P, ARONSON W J, ZHANG Y J, et al. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland[J]. Journal of Agricultural and Food Chemistry, 2007, 55(19): 7732-7737.
[14]GONZÁLEZ-SARRÍAS A, GIMÉNEZ-BASTIDA J A, GARCÍACONESA M T, et al. Occurrence of urolithins, gut microbiota ellagic acid metabolites, and proliferation markers expression response in human prostate gland upon consumption of walnuts and pomegranate juice[J]. Molecular Nutrition & Food Research, 2010, 54(3): 311-322.
[15]FREEDLAND S J, CARDUCCI M, KROEGER N, et al. A doubleblind, randomized, neoadjuvant study of the tissue effects of POMx pills in men with prostate cancer before radical prostatectomy[J]. Cancer Prevention Research, 2013, 6(10): 1120-1127.
[16]NUNEZ-SANCHEZ M A, GARCIA-VILLALBA R, MONEDEROSAIZ T, et al. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients[J]. Molecular Nutrition & Food Research, 2014, 58(6): 1199-1211.
[17]CERDÁ B, PERIAGO P, ESPÍN J C, et al. Identification of urolithin A as a metabolite produced by human colon microflora from ellagic acid and related compounds[J]. Journal of Agricultural and Food Chemistry, 2005, 53(14): 5571-5576.
[18]GARCÍA-VILLALBA R, BELTRÁN D, ESPÍN J C, et al. Time course production of urolithins from ellagic acid by human gut microbiota[J]. Journal of Agricultural and Food Chemistry, 2013, 61(37): 8797-8806.
[19]GONZÁLEZ-BARRIO R, TRUCHADO P, ITO H, et al. UV and MS identification of urolithins and nasutins, the bioavailable metabolites of ellagitannins and ellagic acid in different mammals[J]. Journal of Agricultural and Food Chemistry, 2011, 59(4): 1152-1162.
[20]BIALONSKA D, KASIMSETTY S G, KHAN S I, et al. Urolithins, intestinal microbial metabolites of pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay[J]. Journal of Agricultural and Food Chemistry, 2009, 57(21): 10181-10186.
[21]VERZELLONI E, PELLACANI C, TAGLIAZUCCHI D, et al. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites[J]. Molecular Nutrition & Food Research, 2011, 55(Suppl 1): 35-43.
[22]LARROSA M, GONZÁLEZ-SARRÍAS A, GARCÍA-CONESA M T, et al. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities[J]. Journal of Agricultural and Food Chemistry, 2006, 54(5): 1611-1620.
[23]GONZÁLEZ-SARRÍAS A, LARROSA M, TOMÁS-BARBERÁN F A, et al. NF-κB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts[J]. The British Journal of Nutrition, 2010, 104(4): 503-512.
[24]GIMÉNEZ-BASTIDA J A, GONZÁLEZ-SARRÍAS A, LARROSA M, et al. Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-α-induced inflammation and associated molecular markers in human aortic endothelial cells[J]. Molecular Nutrition & Food Research, 2012, 56(5): 784-796.
[25]KASIMSETTY S G, BIALONSKA D, REDDY M K, et al. Effects of pomegranate chemical constituents/intestinal microbial metabolites on CYP1B1 in 22Rv1 prostate cancer cells[J]. Journal of Agricultural and Food Chemistry, 2009, 57(22): 10636-10644.
[26]GONZÁLEZ-SARRÍAS A, ESPÍN J C, TOMÁS-BARBERÁN F A, et al. Gene expression, cell cycle arrest and MAPK signalling regulation in Caco-2 cells exposed to ellagic acid and its metabolites, urolithins[J]. Molecular Nutrition & Food Research, 2009, 53(6): 686-698.
[27]KASIMSETTY S G, BIALONSKA D, REDDY M K, et al. Colon cancer chemopreventive activities of pomegranate ellagitannins and urolithins[J]. Journal of Agricultural and Food Chemistry, 2010, 58(4): 2180-2187.
[28]LARROSA M, GONZÁLEZ-SARRÍAS A, YÁÑEZ-GASCÓN M J, et al. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on the phenolic metabolism[J]. Journal of Nutritional Biochemistry, 2010, 21(8): 717-725.
[29]ISHIMOTO H, TAI A, YOSHIMURA M, et al. Antioxidative properties of functional polyphenols and their metabolites assessed by an ORAC assay[J]. Bioscience, Biotechnology, and Biochemistry, 2012, 76(2): 395-399.
[30]HADDAD E H, GABAN-CHONG N, ODA K, et al. Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals[J]. Nutrition Journal, 2014, doi: 10.1186/1475-2891-13-4.
[31]QIU Zhenpeng, ZHOU Benhong, JIN Long, et al. in vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells[J]. Food and Chemical Toxicology, 2013, 59: 428-437.
[32]KALLIO T, KALLIO J, JAAKKOLA M, et al. Urolithins display both antioxidant and pro-oxidant activities depending on assay system and conditions[J]. Journal of Agricultural and Food Chemistry, 2013, 61(45): 10720-10729.
[33]KISS A K, GRANICA S, STOLARCZYK M, et al. Epigenetic modulation of mechanisms involved in inflammation: influence of selected polyphenolic substances on histone acetylation state[J]. Food Chemistry, 2012, 131(3): 1015-1020.
[34]AVIRAM M, DORNFELD L, ROSENBLAT M, et al. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and atherosclerotic apolipoprotein E-deficient mice[J]. The American Journal of Clinical Nutrition, 2000, 71(5): 1062-1076.
[35]VICINANZA R, ZHANG Y J, HENNING S M, et al. Pomegranate juice metabolites, ellagic acid and urolithin a, synergistically inhibit androgen-independent prostate cancer cell growth via distinct effects on cell cycle control and apoptosis[J]. Evidence-Based Complementary and Alternative Medicine, 2013, doi: 10.1155/2013/247504.
[36]STOLARCZYK M, PIWOWARSKI J P, GRANICA S, et al. Extracts from Epilobium sp. Herbs, their components and gut microbiota metabolites of Epilobium ellagitannins, urolithins, inhibit hormonedependent prostate cancer cells-(LNCaP) proliferation and PSA secretion[J]. Phytotherapy Research, 2013, 27(12): 1842-1848.
[37]COZZA G, GIANONCELLI A, BONVINI P, et al. Urolithin as a converging scaffold linking ellagic acid and coumarin analogues: design of potent protein kinase CK2 inhibitors[J]. ChemMedChem, 2011, 6(12): 2273-2286.
[38]FURLANETTO V, ZAGOTTO G, PASQUALE R, et al. Ellagic acid and polyhydroxylated urolithins are potent catalytic inhibitors of human topoisomeraseⅡ: an in vitro study[J]. Journal of Agricultural and Food Chemistry, 2012, 60(36): 9162-9170.
[39]GONZALEZ-SARRIAS A, MIGUEL V, MERINO G, et al. The gut microbiota ellagic acid-derived metabolite urolithin A and its sulfate conjugate are substrates for the drug efflux transporter breast cancer resistance protein (ABCG2/BCRP)[J]. Journal of Agricultural and Food Chemistry, 2013, 61(18): 4352-4359.
[40]GIMÉNEZ-BASTIDA J A, TRUCHADO P, LARROSA M, et al. Urolithins, ellagitannin metabolites produced by colon microbiota, inhibit quorum sensing in Yersinia enterocolitica: phenotypic response and associated molecular changes[J]. Food Chemistry, 2012, 132(3): 1465-1474.
[41]DELLAFIORA L, MENA P, COZZINI P, et al. Modelling the possible bioactivity of ellagitannin-derived metabolites. In silico tools to evaluate their potential xenoestrogenic behavior[J]. Food & Function, 2013, 4(10): 1442-1451.
[42]DENNIS K E, HILL S, ROSE K L, et al. Augmented cardiac formation of oxidatively-induced carbonylated proteins accompanies the increased functional severity of post-myocardial infarction heart failure in the setting of type 1 diabetes mellitus[J]. Cardivascular Pathology, 2013, 22(6): 473-480.
[43]AZORÍN-ORTUÑO M, URBÁN C, CERÓN J J, et al. Safety evaluation of an oak-flavored milk powder containing ellagitannins upon oral administration in the rat[J]. Journal of Agricultural and Food Chemistry, 2009, 56(8): 2857-2865.
[44]ISHIMOTO H, SHIBATA M, MYOJIN Y, et al. in vivo antiinflammatory and antioxidant properties of ellagitannin metabolite urolithin A[J]. Bioorganic & Medicinal Chemistry Letters, 2011, 21(19): 5901-5904.
YIN Peipei1, YAN Linlin1, CAO Ruoyu1, CHEN Xiaoyuan2, MA Chao1,*, LIU Yujun1,*
(1. College of Biological Sciences and Technology, Beijing Forestry University, Beijing 100083, China; 2. Shandong Institute for Food and Drug Control, Jina n 250101, China)
Abstract: Ellagitannins and ellagic acid are natural polyphenols widely distributed in pomegranate, strawberry, raspberry, blackberry, grape and walnut. However, the absorption of ellagitannins and ellagic acid is extremely poor and the unabsorbed ellagitannins and ellagic acid can be further metabolized to urolithins by the gut microbiota in the colon. It is therefore conceivable that the health effects of ellagitannins-containing products can be associated with the gut-produced urolithins, and thus, the evaluation of biological effects of these metabolites is essential. The present review covers the recent studies concerning the chemical properties and biological activities of urolithins.
Key words: urolithins; ellagic acid; antioxidant; anti-inflammatory; anticancer
doi:10.7506/spkx1002-6630-201507047
中图分类号:TS201.4
文献标志码:A
文章编号:1002-6630(2015)07-0256-05
*通信作者:马超(1979—),男,副教授,博士,研究方向为天然产物化学。E-mail:machao@bjfu.edu.cn刘玉军(1962—),男,教授,博士,研究方向为药用植物学。E-mail:yjliubio@126.com
作者简介:尹培培(1989—),女,硕士,研究方向为药用植物及其次生代谢产物。E-mail:happy62889@126.com
基金项目:北京市高等学校“青年英才计划”项目(YETP0757)
收稿日期:2014-05-22