SHEN Zhuying, HUANG Zhanwang *, XIAO Jianhui, CAO Jingwen
(Jiangxi Key Laboratory of Natural Products and Functional Food, College of Food Science and Engineering,Jiangxi Agricultural University, Nanchang 330045, China)
Abstract:To fully understand the proliferation of splenocyte in vitro of natto ingredients, two kinds of polysaccharideprotein complexes, named as NPPC-1-b and NPPC-2-a, were extracted a nd puri☒ed from natto by DEAE-52 cellulose ionexchange chromatography and Sephadex G-100 gel ☒ltration chromatography. Their molecular weights were determined by performance gel permeation chromatography to be approximately 26.93 and 357.60 kD for NPPC-1-b and NPPC-2-a,respectively. The two complexes were further characterized by FT-IR, UV-visible spectrophotometry, monosaccharide and amino acid composition analysis. The results showed that NPPC-1-b contained an O-glycopeptide bond, while NPPC-2-a contained an N-glycopeptide bond. NPPC-1-b was mainly composed of mannose (Man), rhamnose (Rha)and arabinose (Ara), with the contents of 67.45%, 25.67% and 2.44%, respectively. However, NPPC-2-a was mainly composed of glucose (Glc), mannose (Man), arabinose (Ara), xylose (Xyl) and galactose (Gal), accounting fo r 36.64%,21.71%, 10.35%, 9.00% and 5.17%, respectively. Seventeen amino acids were identified in both complexes. NPP C-1-b was mainly composed of glutamic (Glu), proline (Pro), lysine (Lys) and arginine (Arg) with contents of 0.971%, 0.791%,0.708% and 0.603%, while NPPC-2-a was mainly composed of Glu, Pro and aspartic (Asp) acid with contents of 0.903%,0.689% and 0.504%. Besides, preliminary in vitro tests showed that NPPC-1-b (100-200 μg/mL) and NPPC-2-a (12.5-200 μg/mL) exhibited significantly higher immunomodulatory activities against the proliferation of lymphocytes(P < 0.05), and the effect of 100 μg/mL NPPC-2-a was the best. In respect of cytokines expression, NPPC-1-b and NPPC-2-a significantly enhanced the expression of interleukin-2 (IL-2) and interferon-γ (INF-γ), and 50 μg/mL NPPC-1-b showed a strong effect on IL-2 secretion, 200 μg/mL NPPC-2-a exhibited a strong effect on INF-γ secretion. These results suggest that NPPC-1-b and NPPC-2-a can effectively increase immunomodulatory activity.
Key words:natto; polysaccharide-protein complexes; structure analysis; immunomodula tory activity
In recent decades, glycoprotein have emerged as an important class of bioactive natural products extracted from natural resources, such as bacteria, edible fungi, plant, marine☒ora and fauna. A glycoprotein is a glycoconjugate in which a protein carries one or more carbohydrate chains covalently attached to its polypeptide backbone [1], usually via N- or O-linkages. Increasing attention is being directed towards them, due to their diverse biological functions without side effects [2]. Amon g them, the immunomodulatory and antitumor effects are most reported. For example, polysaccharideproteins from Hirsutella sinensis, polysaccharopeptide and Cyclocarya paliurus were all proved to have the immune and antitumor functions. It is said that the biological activities of polysaccharide-protein might depend on either the protein part or the carbohydrate part, so their unique chemical properties and structural features such as molecular weight,chemical component, glycopeptide bonds attract a great deal of attention.
“Natto” is a traditional Japanese fermented food made by fermenting boiled soybeans with Bacillus subtilis natto. Natto showed many health-promoting activities, such as dissolving thrombus [3], anti-tumor [4], anti-hypertensive [5]and preventing osteoporosis [6]. Many bioactive components compounds,which are responsible for these pharmacological effects,have been identified and isolated from natto. These include nattokinase, angiotensin converting enzyme inhibitors, VK 2,surfactin isoflavone, as well as poly-γ-glutamic acid. Though,numerous attentions were given to natto, the research on glycoconjugates, as composed by proteins/peptides and polysaccharides were rare.
The purpose of this work was to characterize two polysaccharide-protein complexes (NPPC) extracted and puri☒ed from natto, then preliminary discussion for their immunomodulatory effects on splenocyte proliferation and cytokines expression. It may contribute some information to immunomodulatory mechanism of polysaccharide-protein complexes.
1.1 Materials, animals and reagents
The strain of Bacillus subtilis natto was purchased from China Center for Type Culture Collection (Wuhan, China);soybeans were purchased from Wal-Mart supermarket(Nanchang, China).
Male Kunming mice (weighing (20 ± 2) g) were purchased from Hunan SJA Laboratory Animal Co. Ltd.(Hunan, China).
Lipopolysaccharide (LPS), concanavalin A (ConA),methyl thiazolyl tetrazolium (MTT), fetal bovine serum(FBS), medium RPMI-1640 were purchased from Biological Industries Co. Ltd. (Israel); enzyme-linked immunosorbent assay (ELISA) kits were obtained from Qisong Co. Ltd.(Beijing, China); DEAE-52 cellulose, Sephadex G-100 and dialysis membranes (flat width 44 mm, M w8 000−14 000 D)were obtained from Solarbio Co. Ltd. (Beijing, China); all other reagents were of analytical grade and purchased from Fucheng Co. Ltd. (Tianjin, China).
1.2 Methods
1.2.1 Preparation of NPPC
Briefly, the boiled soybeans were fermented with Bacillus subtilis natto train at 37 ℃ for 24 h. Then the finished natto product was lyophilized, grounded and d efatted with petroleum ether at 40 ℃ for 12 h.
The degreased natto powders were dried and extracted with distilled water (1∶24, m/V) with ultrasonic treatment(108 W for 40 min). The aqueous extract was centrifuged at 3 900 × g for 20 min. The supernatant was precipitated with 95% ethanol and natural subsidented at 4 ℃ for 24 h. After centrifugation, the obtained precipitate was dissolved in Sevag reagent (1-butanol-chloroform (1:4, V/V)) to remove free protein [7], and exhaustive dialyzed with distilled water for 3 days to remove the low-molecular-weight compounds. Finally the dialyzed supernatant was lyophilized to give crude NPPC, and was stored at -20 ℃ for further puri☒cation.
1.2.2 Puri☒cation of NPPC
The crude NPPC (100 mg) was dissolved in double-distilled water (10 mL) and filtrated (3 μm; Millipore). 5 mL of the filtrate was applied to a ion-exchange system(DEAE-52 cellulose, 1.6 cm × 33 cm, 50 μm), and then eluted with different concentrations of stepwise NaCl solution (0.05, 0.1, 0.2, 0.4, 0.5 mol/L) at a flow rate of 0.5 mL/min. Each tube of 3 mL was automatically collected by the DBS-100-LCD automated fraction collector (Shanghai,China), and the total carbohydrate of each tube was determined by phenol-sulfuric acid method [8]and the protein was monitored by spectrophotometer at 280 nm. As a result,NPPC was resolved into two appropriate fractions (NPPC-1 and NPPC-2) according to the detection result of total carbohydrate and protein. Then, the main part of each fraction was collected, concentrated, lyophilized and further purified through a gel ☒ltration chromatography of Sephadex G-100(1.6 cm × 33 cm, 40−120 μm) with 0.02 mol/L Tris-HCl(pH 7.2) at a flow rate of 0.5 mL/min. The effluent also was collected automatically (3 mL/tube), and the fractions obtained were detected with the same methods as above mentioned. Finally, the resulted s ub-fractions(NPPC-1-a, NPPC-1-b and NPPC-2-a) were collected,dialyzed and lyophilized for further study, respectively.
1.2.3 Effects of NPPC-1-a, NPPC-1-b and NPPC-2-a on the splenocyte proliferation
Splenocyte proliferation assay of the three sub-fractions were performed by MTT method. Briefly, the female Kunming mice were sacri☒ced and submerged in 75% alcohol to sterilize. The spleen was obtained under aseptic conditions,homogenized and sieved through a 200-mesh tainless steel to obtain single cell suspension. After centrifugation, the splenocyte were suspended in the buffer of Tris-NH 4Cl(0.83%) and gently vibrated for 2−3 min to remove red blood cells. After washing twice with PBS buffer (0.1 mol/L,pH 7.2), the splenocyte was re-suspended in complete RPMI-1640 medium including 10% fetal calf serum, 100 U/mL penicillin and 100 μg/mL streptomycin. Cell concentration was adjusted to 5 × 10 6cells/mL and the viability of splenocyte was always over 95% excluded by trypan blue dye.
The splenocyte suspension was seeded into a 96-well cell culture plate and purified NPPC sub-fractions (NPPC-1-a,NPPC-1-b and NPPC-2-a), prepared in culture medium, with various concentrations (0, 12.5, 25, 50, 100 and 200 μg/mL)were added and giving a final volume of 100 μL. ConA(5 μg/mL) or LPS (10 μg/mL) were used as positive control,whereas complete RPMI-1640 medium alone served as blank control. After incubated for 68 h at 37 ℃ in a humidi☒ed 5% CO 2incubator (Mode 3121, Thermo Labsystems),10 μL of MTT solution (5 mg/mL) was added to each well and incubated for another 4 h again. Then the culture media were removed and the insoluble dye crystal was allowed to dissolve in 100 μL dimethyl sulfoxide (DMSO) at room temperature. Absorbance at 570 nm was determined by Multiskan MK3 ELISA reader (Thermo Labsystems). The sample group was compared with control group and the stimulation index (SI) was calculated according to the following equation.
Where A exand A cowere the absorbance of experimental and control group, respectively.
1.2.4 Preliminary characterization of NPPC-1-b and NPPC-2-a
1.2.4.1 Composition, homogeneity and molecular weight
The total content of carbohydrate was determined by the phenol-sulphuric acid assay, and the content of protein was measured by the coomassie brilliant blue G-250 method [9].
The homogeneity and molecular weight were identified by high-performance gel permeation chromatography(HPGPC) on an Agilent Technologies 1200 system (PL Aquagel-OH Mix column(300 mm × 7.8 mm, 8 μm); a BI-M wA; a UV detector and a differential refractometer). 50 μL of sample solution was ☒ltered through a 0.22 μm membrane and injected in each run. NaNO 3including 0.02% NaN 3was used as the eluent buffer at a flow rate of 0.7 mL/min. The column was maintained at 35 ℃, and the data was analyzed by Par SEC workstation. The average molecular weight of NPPC-1-b and NPPC-2-a were estimated by reference to the calibration curve made from a set of dextran standards (M w4 320−289 000 kD).
1.2.4.2 Amino acid and monosaccharide composition
The amino acid composition was determined by Hitachi L-8800 Amino A cid Auto Analyzer [10]. NPPC-1-b and NPPC-2-a were hydrolyzed in vacuum with 6 mol/L HCl at 110 ℃ for 24 h in a sealed tube.
The monosaccharide was analyzed by an Agilent 6890N GC-MS system equipped with a HP-5capillary column(30 m × 0.25 mm, 0.25 μm) and ☒ame-ionization detector(FID). Briefly, 10 mg of NPPC-1-b or NPPC-2-a was hydrolysed with 2 mol/L trifluoroacetic acid (TFA) at 110 ℃ for 6 h in a sealed tube. After removing the residual acid with methanol under reduced pressure, the hydrolysate was converted to acetylated aldononitrile derivatives bysuccessively processed with hydroxylamine hydrochloride,pyridine and acetic anhydride. Standard sugars were converted to their acetylated derivatives according to the above-mentioned method. The oven temperature was maintained at 70 ℃ for 3 min, and to 180 ℃ at 10 ℃/min for 5 min, then increased gradually to 250 ℃ at a rate of 3 ℃/min and finally held at 250 ℃ for 5 min.
1.2.4.3 Glycosidic linkage
The samples of NPPC-1-b and NPPC-2-a were incubated with 0.2 mol/L NaOH at 45 ℃ for 2 h. The solutions were measured under the wavelength ranged from 200 to 400 nm by the SPECORD200 ultraviolet spectrophotometer (Jena analysis instrument Co. Ltd., German). The sample without alkali treatment was the control [11].
1.2.4.4 Infrared spectroscopy
Infrared spectra of NPPC-1-b and NPPC-2-a were recorded on a Nicolet 5700 FT-IR spectrophotometer(ThermoElectron, Madison, WI, USA) in the range of 4 000−400 cm -1with the resolution of 2 cm -1. 1 mg of dry sample and potassium bromide (100 mg) were incorporated,grounded and pressed into a 1 mm disk for spectrum recording at room temperature [12].
1.2.5 Effects of NPPC-1-b and NPPC-2-a on the production of cytokines in the splenocyte supernatant
Cultural method of splenocyte cell is same as 1.2.3. The concentrations of IL-2, IL-10 and INF-γ in each splenocyte culture supernatant were assayed by using ELISA kits.
1.3 Statistics analysis
All the figures were created using Origin 8, the data were expressed as the,
 and subjected to one-way ANOVA using SPSS system (version 17.0). Means were separated by Fisher's protected least significant difference (P < 0.05 or P < 0.01).
and subjected to one-way ANOVA using SPSS system (version 17.0). Means were separated by Fisher's protected least significant difference (P < 0.05 or P < 0.01).
2.1 Isolation and puri☒cation of NPPC
The crude NPPC was extracted from natto and the total yield was 1.84%. As shown in Fig.1 , after purified by DEAE-52 cellulose column, 5 fractions based on the tot al carbohydrate elution profile were obtained. Among them, NPPC-1 and NPPC-2, eluted by 0.05 and 0.1 mol/L NaCl solution, respectively, simultaneously showed weak absorbance at 280 nm. Since the crude NPPC was treated several times by the Sevag method to remove free protein, NPPC-1 and NPPC-2 might be two kinds of acidic polysaccharide-protein complexes.
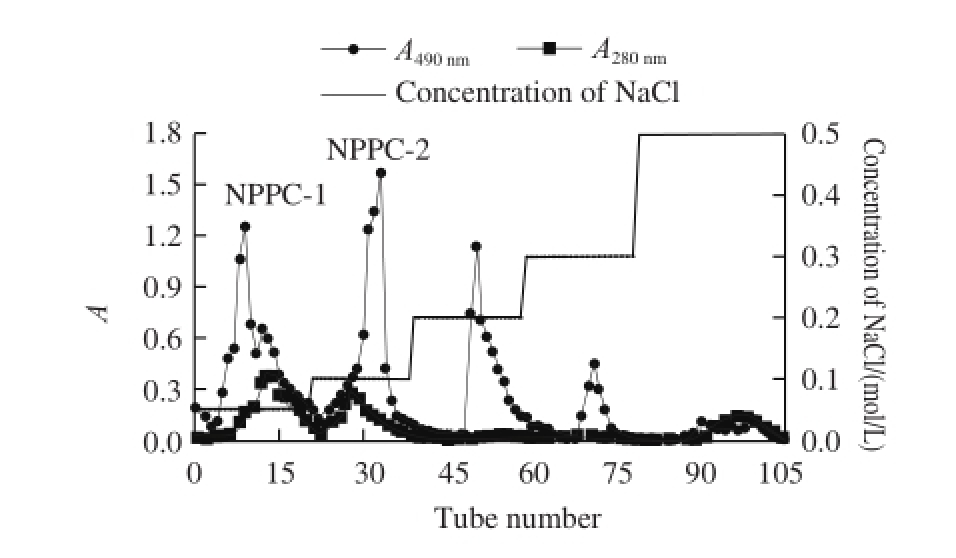
Fig.1 DEAE-52 cellulose chromatography profile of crude NPPC
After concentrating and lyophilizing, NPPC-1 and NPPC-2 were further purified by Sephadex G-100 gel☒ltration chromatography. As a result, NPPC-1 generated two sub-fractions (NPPC-1-a and NPPC-1-b) based on the elution profile of total carbohydrate and protein (Fig.2 A), while NPPC-2 yielded only one main overlapping peak named as NPPC-2-a (Fig.2 B). Then collected and lyophilized these three extracts, which accounted for 10.36%, 15.58% and 19.27% of crude NPPC, respectively.
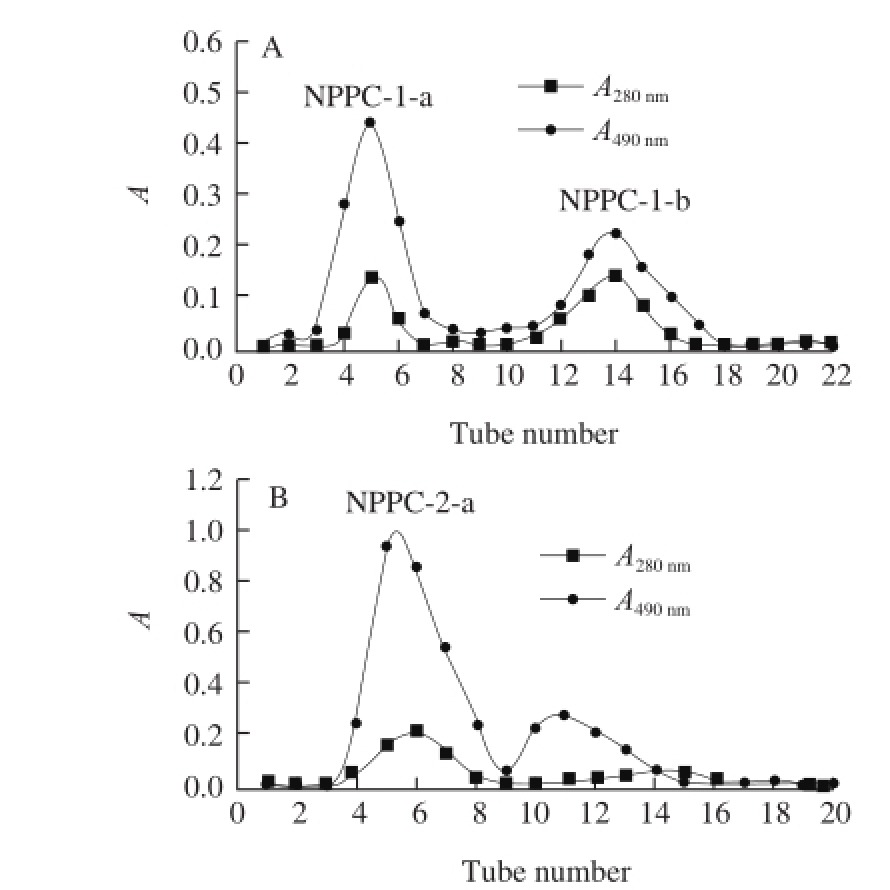
Fig.2 Sephadex G-100 chromatography profiles of NPPC-1(A) and NPPC-2(B)
2.2 Effects of NPPC-1-a, NPPC-1-b and NPPC-2-a on the splenocyte proliferation
Lymphocyte proliferation is a crucial cellular response in the activation cascade of both cellular and humoral immune responses [13]. In this study, all these three sub-fractions have been screened for their immunomodulation effects on lymphocyte proliferative response. As shown in Fig.3 , all these three sub-fractions could stimulate splenocyte proliferation, but the stimulation ratios of them weresigni☒cantly different. NPPC-2-a, exhibited the strongest proproliferation activity, and had higher stimulation ratios at all the concentrations. Characteristically, dose-dependency was not significant for NPPC-2-a, and it showed maximum stimulatory proliferation activity at 100 μg/mL, the reason might be due to the strong stimulatory proliferation activity of NPPC-2-a, which induced a strong proliferation on the splenocyte at low concentrations. Although the proliferation indices of NPPC-1-b were less than NPPC-2-a, it also exhibited pronounced activity at high concentrations (100−200 μg/mL). However, compared with the NC group, NPPC-1-a did not show significant effect at all tested concentrations. Thus, NPPC-1-a was not further investigated in the present study.
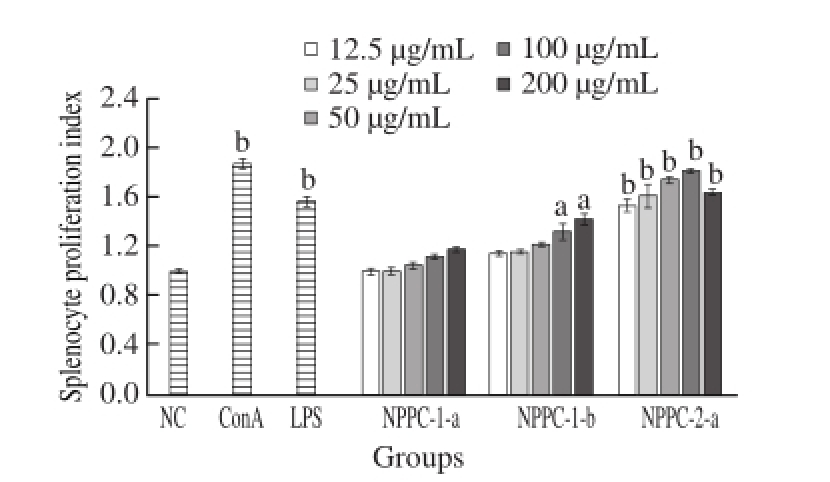
Fig.3 Effects of NPPC sub-fractions on the proliferation splenocytes
2.3 Composition, homogeneity and molecular weight of NPPC-1-b and NPPC-2-a
Table1 Appearance, carbohydrate contents, protein contents, sugar components and molecular weights (M w) of NPPC-1-b and NPPC-2-a
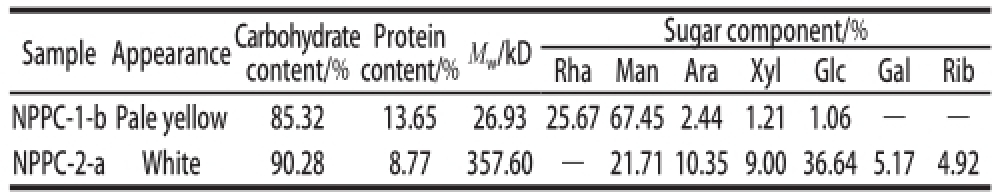
Note: —. It was not detected; Rha. rhamnose; Man. mannose; Ara. arabinose;Xyl. xylose; Glc. glucose; Gal. galactose; Rib. ribose.
content/%M w/kDSugar component/% RhaManAraXylGlcGalRib NPPC-1-b Pale yellow85.3213.6526.93 25.67 67.45 2.441.211.06——NPPC-2-aWhite90.288.77357.60—21.71 10.35 9.00 36.64 5.174.92 Sample AppearanceCarbohydrate content/% Protein
As shown in Table 1, the total sugar content in NPPC-1-b and NPPC-2-a were 85.32% and 90.28%, and the amounts of protein were 13.65% and 8.77%, respectively.
HPGPC was used to determined the purity and molecular of NPPC-1-b and NPPC-2-a. Fig.4 A and 4B illustrated the RI- and UV280 nm-detected chromatograms for NPPC-1-b and NPPC-2-a, respectively. B oth NPPC-1-b and NPPC-2-a showed only one main single and symmetrical peak on HPGPC, and the protein and sugar profile appeared at a similar elution volume, s uggesting that NPPC-1-b and NPPC-2-a were homogeneous protein-bound polysaccharides, and the purity of them were both > 98% based on the phenol-sulfuric acid method and the coomassie brilliant blue G-250 method.
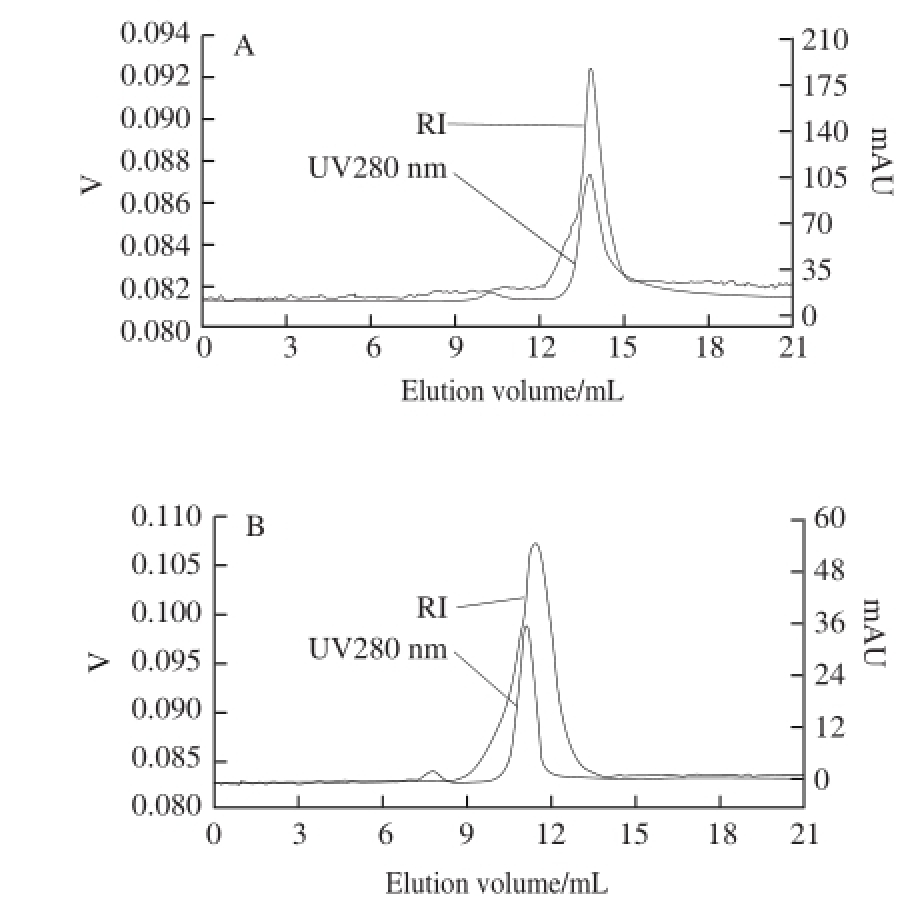
Fig.4 HPGPC profiles of NPPC-1-b (A) and NPPC-2-a (B)
According to the calibration curve with standard dextrans, the molecular weight of NPPC-1-b and NPPC-2-a were calculated to be 26.93 and 357.60 kD, respectively.
Molecular weight was regarded as one of the most important factor related to biological activities of polysaccharides. It have been reported that polysaccharides from Alchornea cordifolia with relatively high-molecular weight were highly active in biological tests with phagocytes,whereas the low-molecular weight sub-fraction was inactive or minimally active in the same cells [14]. Chen Xiaoyu et al. [15]have reported that the anti-tumor activity of β-D-glucan positively correlated with its molecular weight. Our results that NPPC-2-a showed stronger stimulatory proliferation activity than NPPC-1-b was good in agreement with those reports that higher-molecular weight fraction exhibited the stronger bioactivities.
2.4 Monosaccharide and amino acid composition of NPPC-1-b and NPPC-2-a
Polysaccharides contained neutral or acidic sugar,or conjugated with other components, such as proteins and lipids, exhibit various activities [4,16]. Knowledge of the chemical composition and chain component of the glycoconjugates is important for understanding their application and bioactivities. According to the peak area, the monosaccharide compositions of NPPC-1-b and NPPC-2-a were different. As shown in Table 1, NPPC-1-b was mainlycomposed of Man, Rha and Ara with molar percentages of 67.45%, 25.67% and 2.44%, respectively. NPPC-2-a was composed of Glc, Man, Ara, Xyl, Gal and Rib with content of 36.64%, 21.71%, 10.35%, 9.00%, 5.17% and 4.92%, respectively. These data revealed that the main monosaccharide incorporated in NPPC-1-b and NPPC-2-a were different. In addition, Table2 showed the amino acids composition of NPPC-1-b and NPPC-2-a, seventeen amino acids were identified in both complexes. NPPC-1-b was mainly composed of Glu, Pro, Lys and Arg with the contents of 0.971%, 0.791%, 0.708% and 0.603%, while NPPC-2-a was mainly composed of Glu, Pro and Asp acid with the contents of 0.903%, 0.689% and 0.504%.
Table2 Amino acid contents of NPPC-1-b and NPPC-2-a
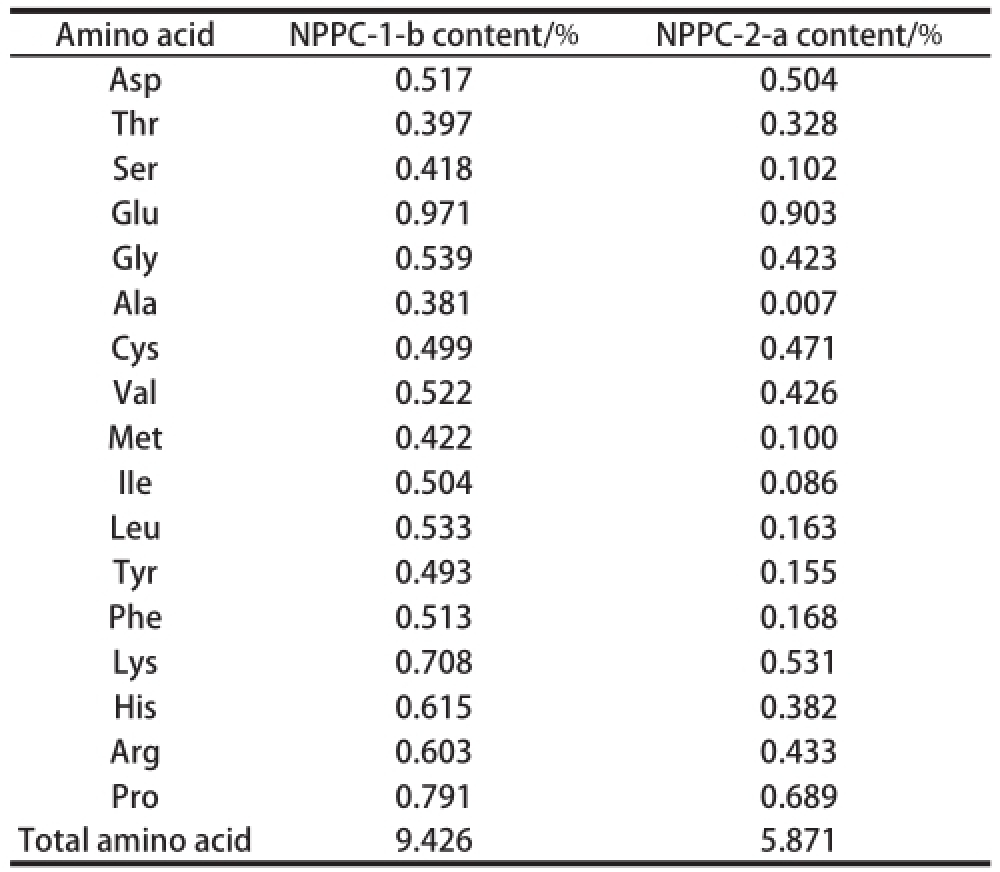
Amino acidNPPC-1-b content/%NPPC-2-a content/% Asp0.5170.504 Thr0.3970.328 Ser0.4180.102 Glu0.9710.903 Gly0.5390.423 Ala0.3810.007 Cys0.4990.471 Val0.5220.426 Met0.4220.100 IIe0.5040.086 Leu0.5330.163 Tyr0.4930.155 Phe0.5130.168 Lys0.7080.531 His0.6150.382 Arg0.6030.433 Pro0.7910.689 Total amino acid9.4265.871
2.5 Linkage analysis of NPPC-1-b and NPPC-2-a
Based on their stability to alkali, there are mainly two types of glycopeptide linkage in polysaccharides-protein(peptide): N-glycopeptide linkage and O-glycopeptide linkage. According to the β-elimination reaction, the N-glycopeptide linkage always keeps stable under low concentration alkaline condition, while the other is easy to be broken and subsequently yield obvious absorption at 240 nm since the serine and threonine of glucosidic bonds translated into α-aminoacrylic acid and α-aminocrotonic acid,respectively. As shown in Fig.5 A and 5B, UV spectra before or after alkali treatment of NPPC-1-b and NPPC-2-a were obtained. Both of them showed no absorption at 260 nm but a weak peak at 280 nm. It can be concluded that there was no nucleic acid, only a little protein, which was in accordance with the results of Fig.4 A and 4B. After alkaline treatment, a stronger absorbance at 240 nm was observed in NPPC-1-b, which suggesting the existence of O-glycopeptide linkage. However, for NPPC-2-a,it could be found that the bond involved between the protein and carbohydrate moiety was N-glycopeptide linkage.

Fig.5 UV spectra of NPPC-1-b (A) and NPPC-2-a (B)
2.6 FT-IR spectroscopy analysis of NPPC-1-b and NPPC-2-a
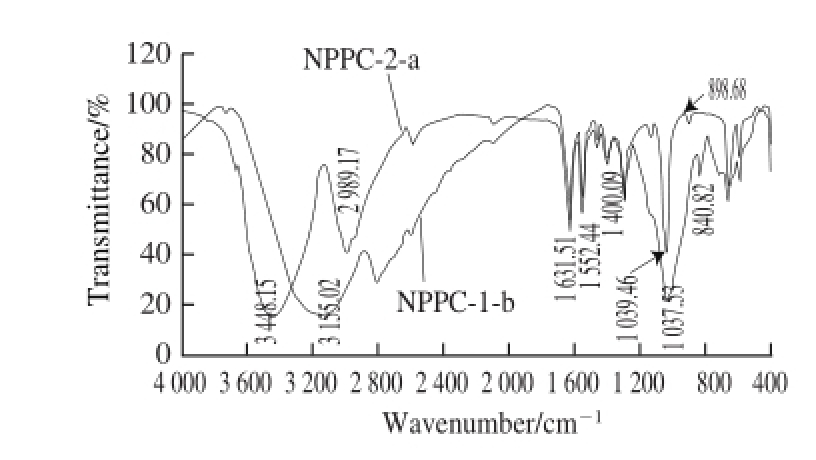
Fig.6 IR spectra of NPPC-1-b and NPPC-2-a
Fig.6 illustrated the IR spectrum of NPPC-1-b and NPPC-2-a. The typical band at 3 155.02 cm -1in NPPC-1-b and 3 448.15 cm -1in NPPC-2-a were corresponding to the hydroxyl group and the hydrogen bond within or between the molecules. The weak band around 2 900 cm -1in NPPC-1-b and NPPC-2-a were due to C—H stretching and bending vibration. Meanwhile, the relatively strong absorption bands at 1 631.51 cm -1and 1 552.44 cm -1for both of them were attributed to the carbonyl bond of the amide group, further indicated the existence of protein [17]. In addition, the strong and symmetrical stretching peak near 1 400 cm -1was an indication of the presence of carboxyl(—COO -) groups [18]. The results are in accordance with the isolation treatment by DEAE-52 cellulose column eluted with NaCl. Besides, the main absorptions of C—O stretching(1 037.53, 1 039.46 cm -1) demonstrated that the characteristics of sugar structures were pyranose con☒guration. As a consequence, there were two types of end carbon-glucoside bonds (α- and β-con☒guration). The typical absorption at 840.82 cm -1indicated that the bond in NPPC-1-b was α-style,while the C—H stretching vibration towards 898.68 cm -1verified the existence of β-con☒guration in NPPC-2-a [19]. 2.7 Effects of NPPC-1-b and NPPC-2-a on cytokine secretion in the splenocyte culture
Except for lymphocyte proliferation, immunomodulatory effects are also generally evaluated by their ability to induce immune cells to secrete immune cytokines [20]. As shown in Fig.7 , the effects of NPPC-1-b and NPPC-2-a on the secretions of IL-2, IFN-γ and IL-10 were determined. IL-2 has many immunomodulatory effects and exhibits high cytotoxic activity against autologous tumour cell. And IFN-γ is the architect behind an amazing immunological program of host resistance to intracellular bacterial and protozoan infections. While IL-10, an immunosuppressive cytokine,could diminish several Th1 cell-mediated responses [21]. In this study, LPS and medium alone were served as the positive and normal control, respectively. The results showed that IL-2 a nd IFN-γ secretions were signi☒ca ntly (P < 0.05) increased by NPPC-2-a and NPPC-1-b treatments (Fig.7 A and 7C),suggesting that NPPC-2-a and NPPC-1-b, seemed as multicytokine inducer, might have some special effect to induce gene expression of various immunomodulatory cytokines. Since IL-2 and IFN-γ were necessary for the development of specific immunity towards a Th1 phenotype, NPPC-2-a and NPPC-1-b could be the good candidates as Th1-type adjuvants. However, NPPC-1-b and NPPC-2-a showed no effect on cytokine IL-10 (Fig.7 B). Although the levels of IL-10 in both treatment groups were higher than normal control group, no significant difference was found (P > 0.05).
NPPC-2-a exhibited the highest IL-2 up-regulation effect at 50 μg/mL, but the best concentration for splenocyte proliferation was 100 μg/mL, which might due to the different surface receptor molecules on cell membrane [22]. In conclusion, based on the splenocyte proliferation, cytokines production and preliminary characterizatiion of NPPC-2-a and NPPC-1-b, it was presumed that the monosaccharide composition, amino acids content as well as molecular weight were crucial for the immunomodulatory activity and its mechanism is worthy of future research.
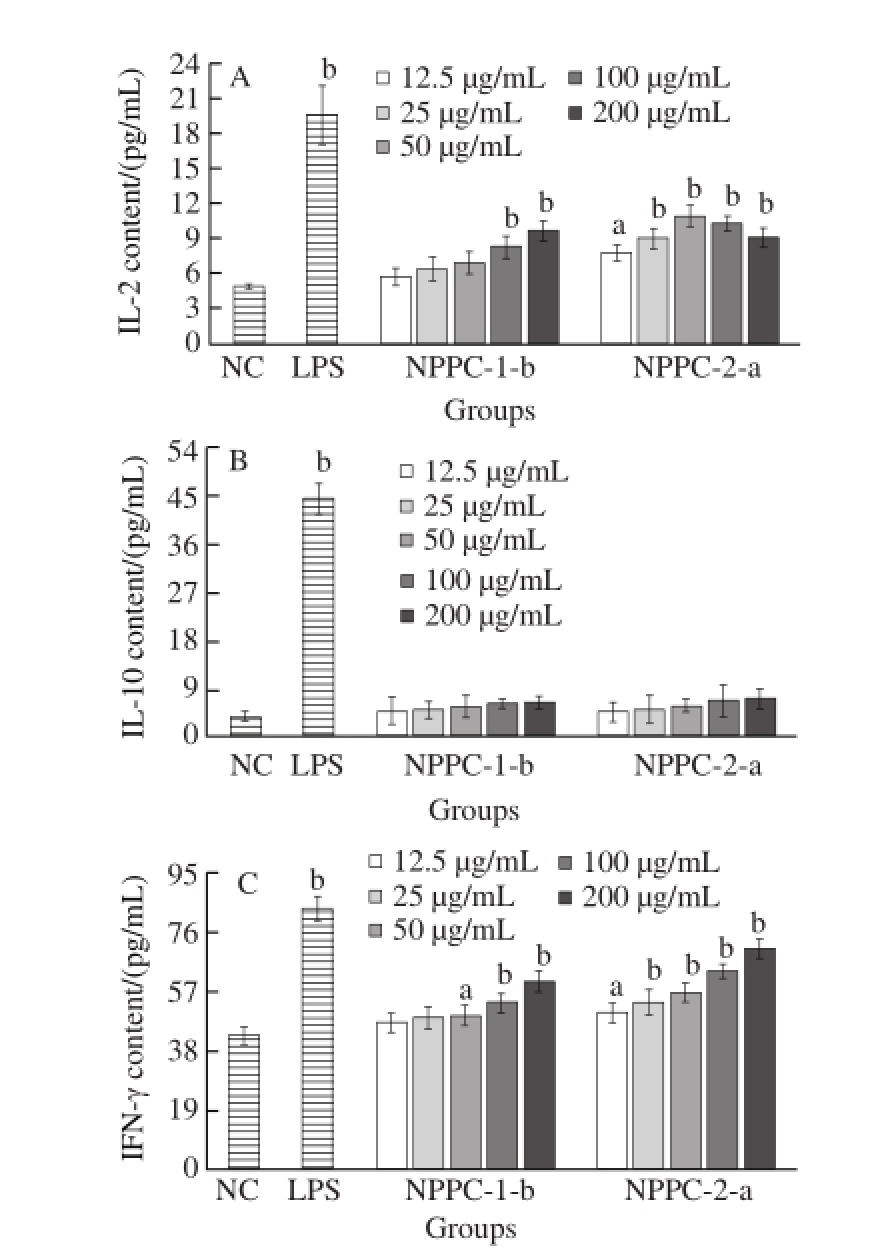
Fig.7 Immunomodulatory effects of three NPPC sub-fractions
A, B, C. Effects on IL-2, IL-10 and IFN-γ secretion in splenocyte culture (± s, n = 4); a. The difference was significant (P < 0.05)when compared with the NC group; b. The difference was very significant (P < 0.01) when compared with the NC group.
Three kinds of natto polysaccharide-protein complexes(NPPC-1-a, NPPC-1-b and NPPC-2-a) were isolated by DEAE-52 cellulose and Sephadex G-100 chromatography in the present study, among them, NPPC-1-b and NPPC-2-a exhibiting a stong activity of splenocyte proliferation in vitro.
It is believed that the immunomodulatory activities of polysaccharide-protein complexes are closely related to structural characteristics, thus partial chemical characteristics of these two polysaccharide-protein complexes were disclosed in our experiment. Finally, The molecular weight of them were calculated about 26.93 and 357.60 kD, respectively. In terms of monosaccharide composition, NPPC-1-b had a large proportion of Man, Rha and Ara, whereas NPPC-2-a had a large proportion of Glc, Man, Ara, Xyl and Gal. The β-elimination reaction revealed that the linkage between carbohydrate and protein was O-glycopeptide bond in NPPC-1-b, while NPPC-2-a was connected with N-glycopeptide bond. Besides the IR spectrum, amino acids composition were also analyzed and compared in thisstudy. In addition, both NPPC-1-b and NPPC-2-a had been demonstrated to have potent immunomodulatory activities,resulting in stimulating the proliferation of splenocyte and secreting the cytokines, such as IL-2 and IFN-γ. Hence,further studies will be focused on the determination of the relationship between their structure and bioactivity and the possible immunoregulation mechanism of NPPC.
References:nces
[1] AJIT V, RICHARD C, JEFFREY E, et al. E ssentials of glycobiology[M]. New York: Cold Spring Harbor Laboratory Press,2009: 107-132.
[2] SCHEPETKIN I A, QUINN M T. Botanical p olys accharides:macrophage immunomodulation and therapeutic potential[J]. International Immunopharmacology, 2006, 6(3): 317-333.
[3] OHSUGI T, SUMIDA E, SUMI H. Effect of a novel substance from natto on tissue-type plasminogen activator (t-PA) release in perfused rat hindlegs[J]. Open Food Science Journal, 2013, 7: 1-5.
[4] BROWN D C, REETZ J. Single agent polysaccharopeptide delays metastases and improves survival in naturally occurring hemangiosarcoma[J]. Evidence-Based Complementary and Alternative Medicine, 2012. doi: 10.1155/2012/384301.
[5] OKAMOTO A, HANAGATA H, KAWAMURA Y, et al. Antihypertensive substances in fermented soybean, natto[J]. Plant Foods for Human Nutrition, 1995, 47(1): 39-47.
[6] FUJITA Y, IKI M, TAMAKI J, et al. Association between vitamin K intake from fermented soybeans, natto, and bone mineral density in elderly Japanese men: the fujiwara-kyo osteoporosis risk in men (FORMEN)study[J]. Osteoporosis International, 2012, 23(2): 705-714.
[7] STAUB A M. Removal of protein: Sevag me thod[J]. Methods in Carbohydrate Chemistry, 1965, 5(2): 5-6.
[8] DUBOIS M, GILLES K A, HAMILTON J K, et al. Colorimetric method for determination of sugars and related substances[J]. Analytical Chemistry, 1956, 28(3): 350-356.
[9] XIE Jianhua, LIU Xin, SHEN Mingyue, et al. Purification,physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves[J]. Food chemistry,2013, 136(3): 1453-1460.
[10] NIE Shaoping, XIE Mingyong, FU Zhihong, et al. Study on the purification and chemical compositions of tea glycoprotein[J]. Carbohydrate Polymers, 2008, 71(4): 626-633.
[11] LI Zhongrui, WANG Bin, CHI Changfeng, et al. Purification and characterization of an antioxidant glycoprotein from the hydrolysate of Mustelus griseus[J]. International Journal of Biological Macromolecules, 2013, 52: 267-274.
[12] WANG Mingchun, JIANG Changxing, MA Liping, et al. Preparation,preliminary characterization and immunostimulatory activity of polysaccharide fractions from the peduncles of Hovenia dulcis[J]. Food Chemistry, 2013, 138(1): 41-47.
[13] SUN Lin, PENG Xiaoxia, SUN Pan, et al. Structural characterization and immunostimulatory activity of a novel linear α-(1→6)-D-glucan isolated from Panax ginseng CA Meyer[J]. Glycoconjugate Journal,2012, 29(5/6): 357-364.
[14] KOUAKOU K, SCHEPETKIN I A, YAPI A, et al. Immunomodulatory activity of polysaccharides isolated from Alchornea cordifolia[J]. Journal of Ethnopharmacology, 2013, 146(1): 232-242.
[15] CHEN Xiaoyu, XU Xiaojuan, ZHANG Lina, et al. Chain conformation and anti-tumor activities of phosphorylated (1→3)-β-D-glucan from Poria cocos[J]. Carbohydrate Polymers, 2009, 78(3): 581-587.
[16] CAO X, WANG A H, JIAO R Z, et al. Surfact in induces apoptosis and G 2/M arrest in human breast cancer MCF-7 cells through cell cycle factor regulation[J]. Cell Biochemistry and Biophysics, 2009, 55(3): 163-171.
[17] CHEN Yi, XIE Mingyong, NIE Shaoping, et al. Purification,composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum[J]. Food Chemistry, 2008,107(1): 231-241.
[18] WU Guanghong, HU Ting, HUANG Zhuolie, et al. Characterization of water and alkali-soluble polysaccharides from Pleurotus tuber-regium sclerotia[J]. Carbohydrate Polymers, 2013, 96(1): 284-290.
[19] FARI☒A J I, VI☒ARTA S C, CATTANEO M, et a l. Structural stability of Sclerotium rolfsii ATCC 201126 β-glucan with fermentation time: a chemical, infrared spectroscopic and enzymatic approach[J]. Journal of Applied Microbiology, 2009, 106(1): 221-232.
[20] LEE J S, SYNYTSYA A, KIM H B, et al. Purific ation, characterization and immunomodulating activity of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.)[J]. International Immunopharmacology, 2013, 17(3): 858-866.
[21] RENNICK D, BERG D, HOLLAND G. Interleukin 10: an overview[J]. Progress in Growth Factor Research, 1992, 4(3): 207-227.
[22] SEN I K, MAJI P K, BEHERA B, et al. Glucan o f a somatic hybrid mushroom, pfls1h: structural characterization and study of immunological activities[J]. International Journal of Biological Macromolecules, 2013, 53: 127-132.
纳豆糖蛋白的分离纯化、结构表征及其免疫活性研究
沈柱英,黄占旺*,肖建辉,曹靖文
(江西农业大学食品科学与工程学院,江西省天然产物与功能食品重点实验室,江西 南昌 330045)
摘 要:为更充分了解纳豆中的成分对脾细胞增殖能力的影响,本实验通过DEAE-52纤维素离子交换柱层析以及Sephadex G-100凝胶柱层析,从纳豆糖蛋白粗提物中获得两种较纯的纳豆糖蛋白:NPPC-1-b和NPPC-2-a。由高效凝胶渗透色谱得到它们的分子质量分别为26.93、357.60 kD。对这两种糖蛋白进行进一步的红外光谱扫描、紫外光谱扫描、单糖组成分析以及氨基酸组成分析显示,NPPC-1-b中含有的是O-糖肽键,而NPPC-2-a中存在的是N-糖肽键。NPPC-1-b主要由甘露糖、鼠李糖、阿拉伯糖组成,且其含量比例分别为67.45%、25.67%和2.44%。NPPC-2-a主要由葡萄糖、甘露糖、阿拉伯糖、木糖以及半乳糖组成,其含量比例分别为36.64%、21.71%、10.35%、9.00%和5.17%。氨基酸自动分析结果显示,两种糖蛋白兼含有人体所需的17 种氨基酸。NPPC-1-b主要由谷氨酸、脯氨酸、赖氨酸和精氨酸组成,其含量分别为0.971%、0.791%、0.708%和0.603%;NPPC-2-a主要由谷氨酸、脯氨酸以及天冬氨酸组成,其含量分别为0.903%、0.689%和0.504%。此外,体外免疫活性探讨发现,100~200 μg/mL的NPPC-1-b以及12.5~200 μg/mL的NPPC-2-a可显著增强脾细胞增殖能力(P<0.05),其中以100 μg/mL的NPPC-2-a作用效果最佳。在细胞因子表达方面, NPPC-1-b和NPPC-2-a可显著促进细胞因子白细胞介素-2(interleukin-2,IL-2)以及干扰素-γ(interferon-γ,IFN-γ)的表达,其中50 μg/mL的NPPC-2-a分泌IL-2最多,200 μg/mL的NPPC-2-a表达IFN-γ的量最高。以上研究结果表明NPPC-1-b和NPPC-2-a具有一定的免疫调节作用。
关键词:纳豆;糖蛋白复合物;结构分析;免疫活性
中图分类号:R284.2;R392.12
文献标志码:A
文章编号:1002-6630(2015)13-0215-08
收稿日期:2014-08-30
基金项目:国家自然科学基金地区科学基金项目(31160337)
作者简介:沈柱英(1988—),女,硕士研究生,研究方向为天然产物的开发与应用。E-mail:zhuyingshen89@163.com
*通信作者:黄占旺(1964—),男,教授,本科,研究方向为天然产物的开发与应用。E-mail:huangzw@163.com
doi:10.7506/spkx1002-6630-201513040