Comparison of Aroma Compounds in Traditional Fermented and Inoculated Douchies, A Chinese Traditional Fermented Soybean Food
YE Yan, SU Wei
*, WANG Qian, HE Xin, GAO Jingya
(College of Life Science, Jiangxi Science and Technology Normal University, Nanchang 330013, China)
Abstract:In this study, cooked soybean was fermented by mixed cultures of Aspergillus oryzae and Aspergillus niger, pure Aspergillus oryzae and the traditional method, respectively. The volatile compound of three Douchi samples was analyzed and compared by gas chromatography-mass spectrometry (GC-MS). A total of 152 volatile compounds, i.e., 30 hydrocarbons, 21 alcohols, 8 aldehydes, 33 esters, 7 ketones, 6 phenols, 18 acids, 20 heterocyclic compounds, 3 sulfocompounds and 6 other compounds, were identified. Results showed that the contents of hydrocarbons, aldehydes and acids in Douchi produced by mixed culture fermentation were higher than in that from pure culture fermentation. The traditional fermented Douchi had higher contents of alcohols, heterocyclic compounds sulfocompounds and phenols when compared with the two other samples. On the other hand, the multi-strains fermented Douchi contained significantly higher amounts of ketones and esters when compared with the pure culture and the traditional fermented Douchi.
Key words:Douchi; fermentation; volatile compounds
叶艳, 苏伟, 王倩, 等. 传统霉菌发酵与接种发酵豆豉风味物质的比较分析[J]. 食品科学, 2016, 37(20): 86-94.
DOI:10.7506/spkx1002-6630-201620015. http://www.spkx.net.cn
YE Yan, SU Wei, WANG Qian, et al. Comparison of aroma compounds in traditional fermented and inoculated Douchies, a Chinese traditional fermented soybean food[J]. Food Science, 2016, 37(20): 86-94. DOI:10.7506/spkx1002-6630-201620015. http://www.spkx.net.cn
Douchi is a Chinese traditional soybean product, particularly in the South China. It has been generally used as a seasoning for food. Like other soybean products, such as natto, tempe and chungkuk-jang, Douchi has been appreciated by consumers as healthy food due to its nutritional contributes and unique flavor and taste
[1]. The traditional preparation method of Douchi is based on the multi-strains fermentation using natural micro-ora, including Saccharomyces and Aspergillus oryzae etc. Recently, increasing attention has been drawn to the pure strain fermentation method, which requires the inoculation of different microbes on the soybeans. Generally, there are four types of Douchi that are produced by pure strain fermentation using Mucor, Rhizopus, Bacteria and Aspergillus. Among them, Aspergillus-fermented Douchi is the most popular one, its production can be traced back at least 2 000 years ago.
Douchi produced by natural fermentation has the characteristic flavor, palatable soft texture, and a bright black color. It usually takes as long as one year for the fermentation process to produce Douchi using traditional method. Compared with naturally fermented Douchi, Douchi produced from pure strain inoculation method requires a much shorter production cycle, which is only about 30 days. However, the quality may not be as good as that of the naturally fermented Douchi. Typically, naturally fermented Douchi has particular aroma, due to the presence of many volatile compounds generated during the long-term fermentation process. In this process, numerous enzymatic and non-enzymatic reactions occur, such as protein degradation, strecker degration and Maillard reactions. These reactions generated various volatile compounds, such as aldehydes, acids, alcohols, ketones, esters, sulphur and many other compounds
[2].
The volatile compounds in various fermented soybean products have been reported previously. For instance, it was found that the predominant volatile compounds in pure Bacillus-fermented included 2,5-dimethylpyrazine, 2-methylbutanoic acid, 2,3,5-trimethylpyrazine, 2-methylpropanoic acid and acetic acid while the major volatile compounds in the naturally fermented soybean included 2,5-dimethylpyrazine, benzaldehyde, 5-methyl-3-hexanone, 2-butanone and 3-methyl-2-pentanone
[3]. Zhao Jianxin et al.
[4]identi ed several aroma active compounds for naturally fermented Chinese soybean paste, including 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone, ethyl linoleate, 2,3-butanediol, acetic acid, fufural, benzene acetaldehyde and pyrazine 2,6-dimethyl. It was detected different major compounds in pure-starter culture natto, which were 2,5-dimethylpyrazine, 2-methylbutanoic acid, acetone, 2,3,5-trimethylpyrazine and 2,3-butanedione
[5-6]. Studies suggest that African soumbala Douchi fermented by pure-starter B. subtilis received preferable scores among various naturally fermented products due to the rich contents of pyrazines and aldehyde
[7]. The correlation between volatile compounds and sensory attributes in Korean Doenjang has also been evaluated by Lee et al.
[8]who found that high furfuryl alcohol and maltol were associated with a sweet-grain attribute. The major classes of volatile compound in three commercial Douchies have been reported as 29 kinds of esters, 18 kinds of acids, 16 kinds of alcohols, 14 kinds of pyrazines, 13 kinds of ketones, 12 kinds of aldehydes, 6 kinds of phenols, 5 kinds of hydrocarbons, 5 kinds of furans, 5 kinds of sulphur-containing compound, 4 kinds of pyridines, 2 kinds of pyrimidines, and 2 kinds of miscellaneous compound
[9].
Although there have been numerous reports about profiles of volatile compounds of traditional fermented soybean. For the sake of improving the aroma of Douchi, the aroma active compounds in traditionally fermented Douchi need to be identi ed. The objective of this study was to compare the volatile compounds in three Douchi samples produced by three different methods such as traditionally fermented Douchi, multi-strains (Aspergillus oryzae and Aspergillus niger), and pure Aspergillus oryzae, respectively, aiming to provide theoretical basis for the industrialized production of inoculated fermentation.
1 Materials and Methods
1.1 Materials and reagents
Aspergillus oryzae 3.042 and Aspergillus niger 3.350 were obtained from China Center of Industrial Culture Collection. Black soybeans was obtained from Jiangxi NanKo Co. Ltd.. Dichloromethane, ether, anhydrous sodium sulfate(all analytically pure) were purchased from Tianjin Chemical Reagent Factory. High purity nitrogen was obtained from Nanchang Grand Gas Co. Ltd..
1.2 Instruments and equipment
523-Nickerson Type SDE Apparatus was obtained from Anhui Dongguan Instrument Co. Ltd.. 6890N/5973I-GCMS and HP-5M (30 m × 0.25 mm, 0.25 μm) were purchased from American Agilent Co. Ltd.. SI114-Electronic analytical balance model was obtained from Hua Hong Instrument Co. Ltd.. 722S-Visible spectrophotometer was obtained from Shanghai Lengguang Technology Co. Ltd..
1.3 Methods
1.3.1 Strains and culture condition
These strains routinely stored on nutrient agar and maintained at -20 ℃ was isolated in 20% glycerol. For preparation of the inoculum, the strains were cultivated in czapek's medium at 37 ℃ for 72 h. The cells were subsequently harvested, suspended with sterile distilledwater and carefully adjusted to achieve a concentration of 10
6CFU/mL. The suspension was used as the inocula for soybean fermentation.
1.3.2 Preparation of Douchi
Protein-rich black soybeans, supplied by local manufacturing company, were used as raw material of Douchi Soybeans. Soybeans were washed and soaked in tap water for 1.5 h at 35 ℃. Soybeans were then boiled for 2 h, cooled to 30-35 ℃, and inoculated immediately with 10
6CFU (per gram of sterilized soybeans) of Aspergillus oryzae 3.042 starter culture. Subsequently, the inoculated soybeans were incubated at 30 ℃ for 72 h under 80% relative humidity in an incubator. In contrast, for multi-strains fermentation, cooked and cooled soybeans were inoculated with 10
6CFU/g of sterilized soybeans) of multi-strains(Aspergillus oryzae 3.042: Aspergillus niger 3.350 of 1:1), incubated at 30 ℃ for 48 h, under 80% relative humidity in an incubator. Semi-finished products were called the Douchiqu(koji). The Douchiqu were salted as far as the content of NaCl reached about 8% (m/m). The sample was ripened for four weeks at 45-50 ℃ in the same incubator. The suffi cient fermented soybeans were pulverized and frozen at -20 ℃ for further research.
1.3.3 Collection of volatile component
A Likens-Nickerson type SDE apparatus was used to collect the volatile compounds. Sample (100 g each) was dissolve by 200 mL of distilled water and the aliquot was loaded in a 500 mL fl ask. Five mililitres of internal standard(IS) [2-methyl-1-pentanol (10 μg/mL in methanol)] was added to the sample before extraction. Each sample was extracted with 100 mL of redistilled dichloromethane and carried out for 4 h till the distilled water started to boil. The extraction was dried over Na
2SO
4overnight and concentrated to 2 mL using a rotor vapor equipped with a water bath. The temperature of the bath water was 35-36 ℃ and the reducing pressure was 13 300-19 950 Pa. The solvent was further removed under a purifi ed nitrogen stream to 1 mL ultimately. The concentrated extraction was stored at -20 ℃ for further analysis.
1.3.4 Gas chromatography-mass spectrometry (GC-MS)analysis
GC operating conditions were: column HP-5MS (30 m × 0.25 mm, 0.25 μm), the column temperature programmed from 70 ℃ isothermal for 2 min, then increased to 200 ℃ at a rate of 3 ℃/min and held isothermal for 15 min. Carrier gas was heliumat flow rate 1 mL/min. Injector temperature was 250 ℃. Volume 1 μL was injected and split ratio was 1:50. Mass spectrometry conditions were: ionisation voltage 70 eV, ion source temperature 280 ℃, and mass scan range 30-450 mass units.
1.3.5 Identification and quantification of volatile compounds
The GC-MS was calibrated daily by running 0.1 μL of a 100 × 10
-6standard mixture of C
5-C
25n-alkanes. Qualities of volatile compounds were identi ed by comparing linear retention indices (LRI) with those standard compounds and mass spectra of compounds by comparison with the bibliographic data of known compounds from the mass spectral database. The quantities of each compound were determined by comparison of the area to the integrated of peaks of the total ion chromatogram and count by comparing peak area with that of the 1,2-dichlorobenzene internal standard.
1.4 Statistical analysis
All data were subjected to analysis of variance(ANOVA), and Duncan's multiple range test (DMRT) was used to compare significant difference of means at P ≤ 0.05. Most experiments were performed in triplicate.
2 Results and Analysis
Similar volatile compounds were identified in the chromatograms of the traditional fermented Douchi, multistrains fermented Douchi and traditional fermented Chinese soybean pastes
[10-11]. However, the difference in the peak intensity of the individual volatiles was found in present study. The number of peaks in the pure Aspergillus oryzae fermented Douchi was less than the other two. The identified volatile compounds were shown in Table 1-9. Variation in the volatile compounds was not only appeared in the inoculated and traditional fermented samples, but also in the natural fermented samples. The Douchi inoculated pure Aspergillus oryzae had the least amounts of volatile compounds, whereas the traditionally fermented samples had the most abundant volatile. A total of 152 compounds were identified from the three samples, including 30 hydrocarbons, 8 aldehydes, 21 alcohols, 33 esters, 7 ketones, 18 acids, 6 phenols, 20 heterocyclic compound, 3 sulfo compound and 6 other compound. Thereinto, esters, alcohols and acids were the major contents, which accounted for approximately 50% of the total volatile compounds. However, only 22 compounds out of 152 were identified in all the samples, which implied that the better process and quality control of the Douchi were needed.
2.1 Hydrocarbons
Table1 GC-MS analysis of hydrocarbons in three different types of Douchi
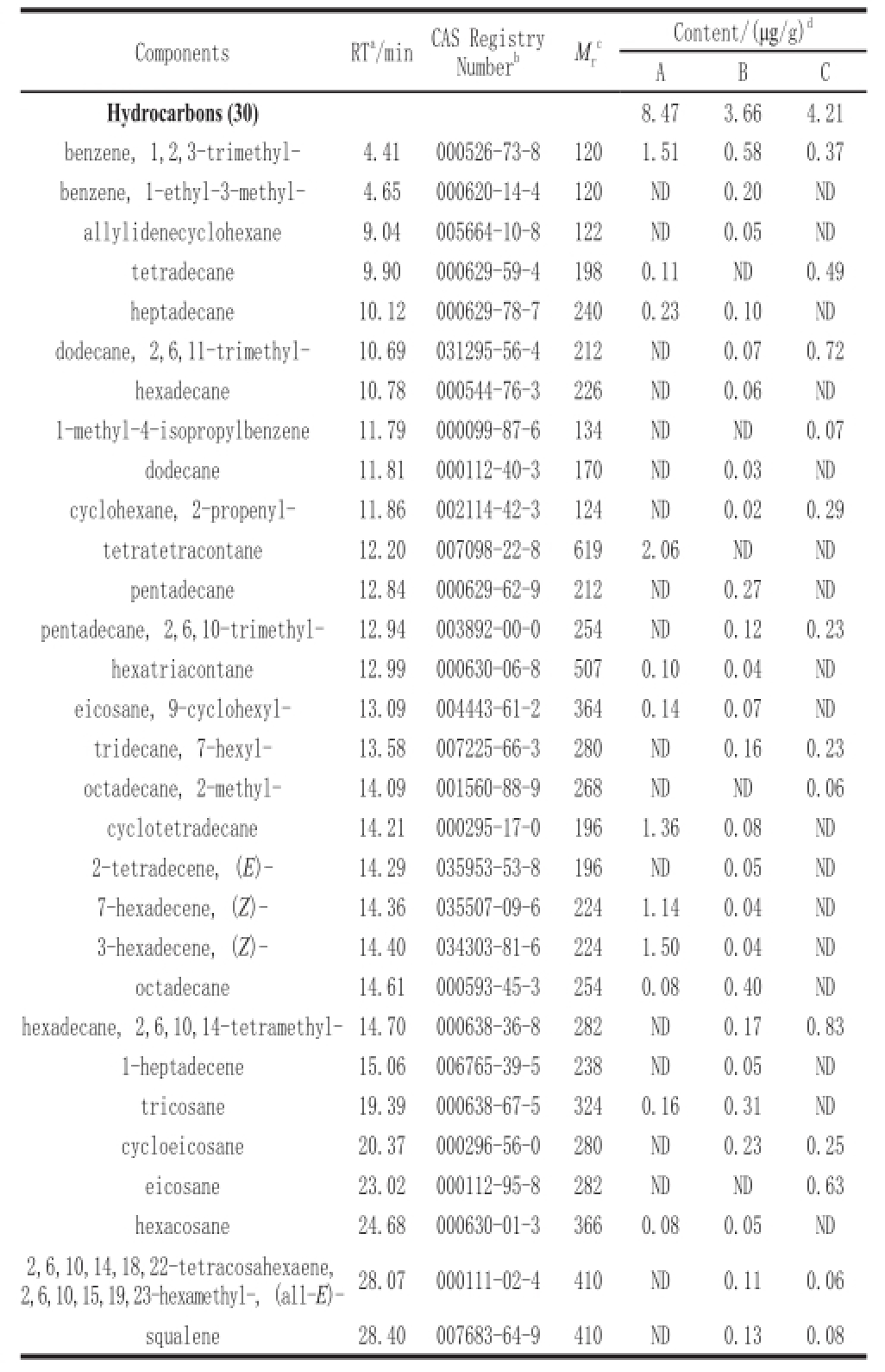
Note: a. retention time; b. chemical abstracts service registry No.; c. relative molecular weight; d. concentration from three replicates on a dry weight basis; A. pure Aspergillus oryzae fermented Douchi; B. traditionally fermented Douchi; C. multi-strains fermented Douchi; ND. not detected or peak area <0.1%. The same is applied in following tables.
Content/(μg/g)
dABC Hydrocarbons (30)8.473.664.21 benzene, 1,2,3-trimethyl-4.41000526-73-81201.510.580.37 benzene, 1-ethyl-3-methyl-4.65000620-14-4120ND0.20ND allylidenecyclohexane9.04005664-10-8122ND0.05ND tetradecane9.90000629-59-41980.11ND0.49 heptadecane10.12000629-78-72400.230.10ND dodecane, 2,6,11-trimethyl-10.69031295-56-4212ND0.070.72 hexadecane10.78000544-76-3226ND0.06ND 1-methyl-4-isopropylbenzene11.79000099-87-6134NDND0.07 dodecane11.81000112-40-3170ND0.03ND cyclohexane, 2-propenyl-11.86002114-42-3124ND0.020.29 tetratetracontane12.20007098-22-86192.06NDND pentadecane12.84000629-62-9212ND0.27ND pentadecane, 2,6,10-trimethyl-12.94003892-00-0254ND0.120.23 hexatriacontane12.99000630-06-85070.100.04ND eicosane, 9-cyclohexyl-13.09004443-61-23640.140.07ND tridecane, 7-hexyl-13.58007225-66-3280ND0.160.23 octadecane, 2-methyl-14.09001560-88-9268NDND0.06 cyclotetradecane14.21000295-17-01961.360.08ND 2-tetradecene, (E)-14.29035953-53-8196ND0.05ND 7-hexadecene, (Z)-14.36035507-09-62241.140.04ND 3-hexadecene, (Z)-14.40034303-81-62241.500.04ND octadecane14.61000593-45-32540.080.40ND hexadecane, 2,6,10,14-tetramethyl-14.70000638-36-8282ND0.170.83 1-heptadecene15.06006765-39-5238ND0.05ND tricosane19.39000638-67-53240.160.31ND cycloeicosane20.37000296-56-0280ND0.230.25 eicosane23.02000112-95-8282NDND0.63 hexacosane24.68000630-01-33660.080.05ND 2,6,10,14,18,22-tetracosahexaene, 2,6,10,15,19,23-hexamethyl-, (all-E)-28.07000111-02-4410ND0.110.06 squalene28.40007683-64-9410ND0.130.08 ComponentsRT
a/minCAS Registry Number
bM
r
c
As shown in Table 1, a total of 30 hydrocarbons were identified from three different samples. The pure Aspergillus oryzae fermented contained 12 hydrocarbons and multistrains fermented Douchi contained 13 hydrocarbons, while traditionally-fermented Douchi contained 25 hydrocarbons, including a large number of alkanes and alkenes. This may be explained by the fact that during traditional fermentation process, some spices were usually added, such as ginger, pepper, garlic, which presumably consisted of many hydrocarbon compounds. Squalene was detected in traditionally fermented and multi-strains fermented Douchi. Squalene, known as the active ingredients of plant oil, has a unique aroma, good oxygen ability, thus anti-hypoxia and anti-fatigue, and enhance human immunity and enhance the function of the gastrointestinal tract
[12].
2.2 Alcohols
Table2 GC-MS analysis of alcohols in three different types of Douchi

Content/(μg/g)
dABC Alcohols (21)10.1719.9719.82 3-hexanol, 2,3-dimethyl-3.74004166-46-5130ND3.124.56 3-pentanol, 3-ethyl-3.75000597-49-91160.643.903.21 9-oxabicyclo[3.3.1]nonan-2-ol9.04133521-31-0142ND0.05ND 2-heptanol, 6-methyl-9.75004730-22-71300.21ND1.13 1-eicosanol10.57000629-96-9298ND0.021.50 1-nonadecanol10.63001454-84-8284ND0.03ND 2-benzylidenecyclohexanol10.89034492-42-7188NDND0.14 benzeneethanol10.9160-12-81220.120.09ND cyclopropanol, 2,2-dimethyl-3-(2-phenylethynyl)-11.221000271-82-1186NDND1.84 tert-hexadecanethiol11.74025360-09-2258ND0.052.37 1-decanol, 2-hexyl-11.78002425-77-6242ND0.530.18 1-tetracosanol11.86000506-51-4354ND0.67ND 1-hexadecanol, 2-methyl-13.09002490-48-42560.87ND0.84 cyclododecanol, 1-ethenyl-14.15006244-49-1210ND4.560.68 E-2-hexadecacen-1-ol14.181000131-10-1240ND0.020.21 ethanol, 2-(tetradecyloxy)-14.21002136-70-12580.160.18ND 2-ethyl-1-dodecanol15.73019780-33-7214ND4.392.26 phytol16.17000150-86-72960.500.960.23 ethanol, 2-(octadecyloxy)-16.64002136-72-33140.150.820.39 1-hexadecanol, 3,7,11,15-tetramethyl-18.18000645-72-72987.52ND0.28(R)-(-)-(Z)-14-methyl-8-hexadecen-1-ol18.92030689-78-2254ND0.58ND ComponentsRT
a/minCAS Registry Number
bM
r
c
Alcohol is the third largest class volatile compound containing 21 different compositionsas shown in Table 2. The 3-pentanol, 3-ethyl-, phytol and ethanol, 2-(octadecyloxy)-were detected in all three Douchi. Alcohols provide pleasant aromas and sweet flavors
[13-14]. Previous researches have shown that the quality of miso due to the alcohol contents
[15-16]. The pure Aspergillus oryzae fermented Douchi contained much less alcohols than the traditionally-fermented and multi-strains fermented Douchi. This was possibly due to the reason that during the fermentation of the pure Aspergillus oryzae Douchi, the temperature was high and the salt tolerant yeast could not grow, resulting in the production of less alcohols. By contrast, for traditionally fermented Douchi, due to long period of fermentation, temperature was low, the salt tolerant yeast had sufficient time to complete alcohols and ester reaction. The multi-strains fermented Douchi exhibited similar profile of alcohols to the traditionally fermented Douchi, probably due to the addition of yeast during postfermentation as well as the lower fermentation temperature.
2.3 Aldehydes
A total of 8 aldehydes were identified from three different samples as shown in Table 3. Benzene acetaldehyde was the most abundant compound in the pure Aspergillus oryzae fermented Douchi, some aldehydes found in Douchiextraction have been reported in other soy products. Benzaldehyde identified in this work was reported in sufu
[17-18], vmiso
[19-20], natto
[21], and soy sauce
[22]. Phenylacetaldehyde found in Douchi extracts was previously reported in sufu, miso, soy sauce, and the unflavoured textures soy protein
[23]. 5-Methyl-2-phenyl-2-hexenal was reported in sufu, the favorable odor of aldehyde compound such as benzaldehyde(cherry or almond-like odor), benzeneacetaldehyde (rosy-like odor) and 2-phenyl-2-butenal (floral, prune-like odor) can be considered to enhance the flavor of Douchi. These aldehydes can be produced by lipid oxidation and degradation during fermenting.
Table3 GC-MS analysis of aldehydes in three different types of Douchi

cContent/(μg/g)
dABC Aldehydes (8)23.71 10.70 13.59 benzaldehyde3.65000100-52-7106ND2.651.59 benzeneacetaldehyde4.98000122-78-112021.303.5012.80 benzeneacetaldehyde, α-ethylidene-8.44004411-89-61410.90NDND 1H-indene-4-carboxaldehyde, 2,3-dihydro-8.44051932-70-8146ND0.20ND benzeneacetaldehyde, α-(2-methylpropylidene)-9.73026643-91-41741.020.070.13 2-furanacetaldehyde, α-isopropylidene-9.80031681-28-4150ND0.58ND 5-methyl-2-phenyl-2-hexenal11.23021834-92-41880.431.160.85 tetradecanal20.20000124-25-42120.061.540.22 ComponentsRT
a/minCAS Registry Number
bM
r
2.4 Ketones
Table4 GC-MS analysis of ketones in three different types of Douchies

cContent/(μg/g)
dABC Ketones (7)1.922.522.97 7-oxabicyclo[4.1.0]heptan-2-one, 4,4,6-trimethyl-6.94010276-21-8154NDND1.35 cyclohexanone, 3,3,5,5-tetramethyl7.12014376-79-51540.30ND1.20 ethanone, 1-(2-hydroxy-5-methylphenyl)-9.34001450-72-21500.190.85ND 4-hydroxy-3-methylacetophenone9.52000876-02-81500.810.64ND 2-benzylcyclohexanone10.91000946-33-81880.62ND0.36 2-cyclopenten-1-one, 4-hydroxy-2-methyl-3-phenyl-11.23069745-73-9ND0.86ND 2-piperidinone, 1-(4-bromobutyl)-13.19195194-80-0234ND0.170.06 ComponentsRT
a/minCAS Registry Number
bM
r
A total of 7 ketones were detected from three different Douchies as shown in Table 4. We identified 7-oxabicyclo, heptan-2-one and 4,4,6-trimethyl only in multi-strains fermented Douchi, 2-cyclopenten-1-one, 4-hydroxy-2-methyl-3-phenyl- was only identified in traditional fermented Douchi, besides we identified ethanone, 1-(2-hydroxy-5-methylphenyl)- and 4-hydroxy-3-methylacetophenone both in pure Aspergillus oryzae fermented and traditional fermented Douchi, 3,3,5,5-tetramethyl cyclohexanone and 2-benzylcyclohexanone were both found in pure Aspergillus oryzae fermented and multi-strains fermented Douchi, 2-piperidinone,1-(4-bromobutyl)- was found in traditional fermented and multi-strains fermented Douchi. Ketones can be formed by fungal enzymatic actions on lipids and/or amino acids, or by the Maillard reaction
[24]. Some ketones such as 4-hydroxy-3-methylacetophenone 2-benzylcyclohexanone were found in Douchi have also been reported in soy sauces
[25].
2.5 Acids
Table5 GC-MS analysis of acids in three different types of Douchi

Content/(μg/g)
dABC Acids (18)42.1930.0527.80 pentanoic acid, 3-methyl-3.17000105-43-11165.742.346.36 3-methyl-2-furoic acid3.71004412-96-8126NDND0.20 2,3,4-trimethylpentanoic acid3.74090435-18-01444.633.622.24 2-butenoic acid, 3-methyl-3.90000541-47-91001.570.03ND 5-benzoylpentanoic acid4.14097547-93-8206ND1.20ND hexanoic acid4.79000142-62-1116ND0.592.33 butanoic acid, 3-methyl-4.96000503-74-2102ND0.271.25 pentadecanoic acid15.36001002-84-22421.581.04ND hexadecanoic acid, 2-methyl-15.93027147-71-32700.32ND0.22 n-hexadecanoic acid16.76000057-10-325614.584.687.84 tetradecanoic acid16.82000544-63-82283.542.510.46 tridecanoic acid16.88000638-53-9214ND4.892.13 n-decanoic acid16.96000334-48-51721.141.97ND 9,12-octadecadienoic acid (Z,Z)-18.20000060-33-32806.445.443.30 1-heneicosyl formate18.22077899-03-73402.65ND0.32 9-undecenoic acid, 2,6,10-trimethyl-18.571000131-86-2226NDND0.18 nonahexacontanoic acid19.73040710-32-5999NDND0.29 22-tricosenoic acid19.78065119-95-1352ND1.47ND ComponentsRT
a/minCAS Registry Number
bM
r
c
Acids containing 18 compounds were the most abundant class in three Douchi as shown in Table 5. 3-Methyl-pentanoic acid, 2,3,4-trimethylpentanoic acid, n-hexadecanoic acid, tetradecanoic acid and 9,12-octadecadienoic acid (Z,Z)-were detected in all three samples. 3-Methyl-2-furoic acid, 2,6,10-trimethyl-9-undecenoic acid and nonahexacontanoic acid were only found in multi-strains fermented samples, 5-benzoylpentanoic acid and 22-tricosenoic acid were only found in traditional fermented samples, 3-methyl-2-butenoic acid, pentadecanoic acid and n-decanoic acid were detected in pure Aspergillus oryzae and traditionally-fermented samples. Hexanoic acid, 3-methyl-butanoic acid and tridecanoic acid were detected in traditional and multi-strains fermented samples. Hexanoic acid was derived from the oxidation of hexanal, has been described as having a “sweat-like” odoras well as possessing a cheesy, fatty, sweaty, sour, rancid and pungent-like odor (11). In general, these acids are described as having cheesy odours, including butanoic acid (cheesy, sharp, rancid, sweaty, sour), 2/3-methylbutanoic acid (cheese, rancid, sweaty) and hexanoic acid (cheesy, fatty, sweaty,sour, rancid, pungent)
[26], and pentanoic acid (sweaty, rancid)mainly contribute oily odours. 2-Methylpropanoic acid and 3-methylbutanoic acid were derived from valine and leucinedegradation
[27].
2.6 Esters
Table6 GC-MS analysis of esters in three different types of Douchies
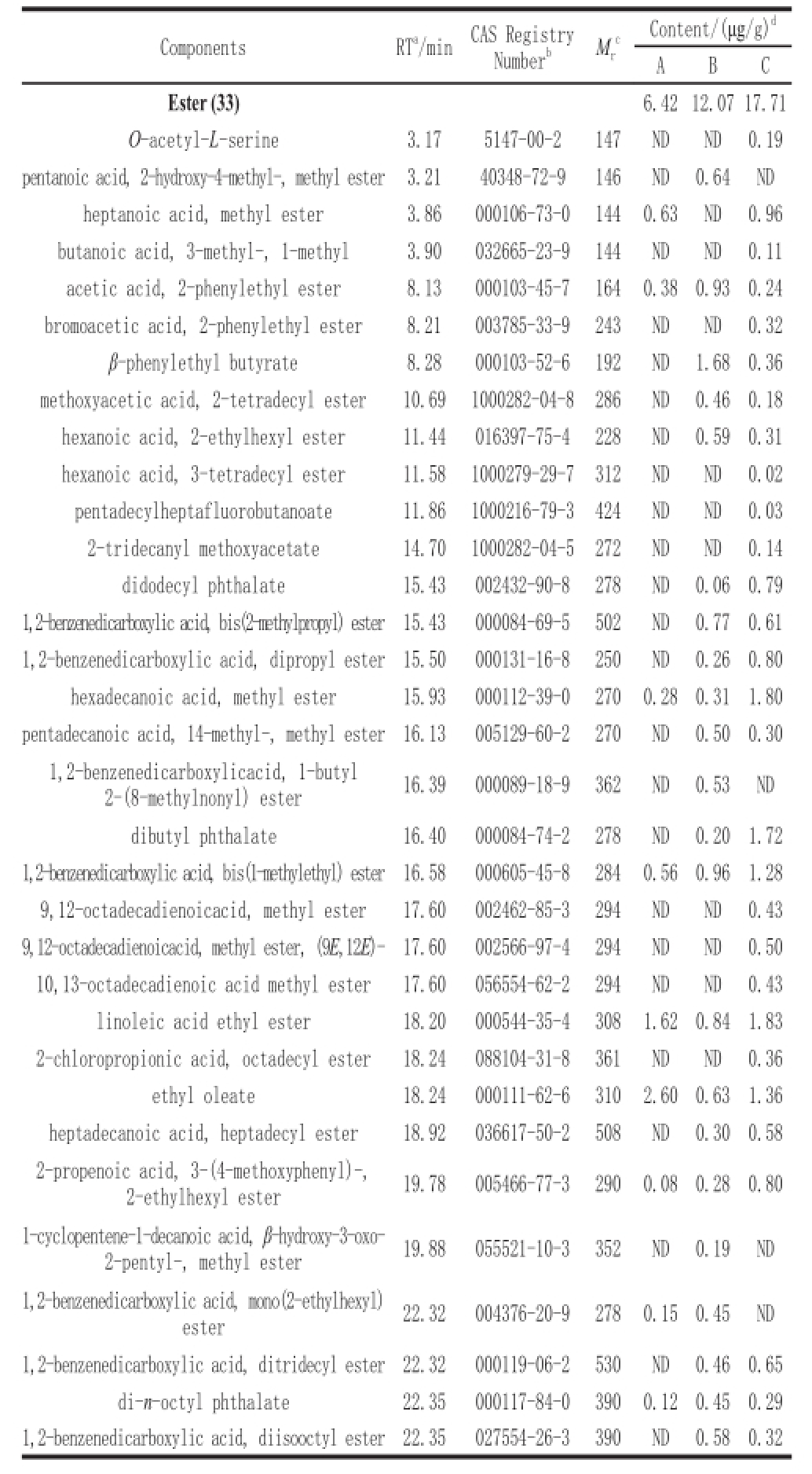
cContent/(μg/g)
dABC Ester (33)6.42 12.07 17.71 O-acetyl-L-serine3.175147-00-2147NDND0.19 pentanoic acid, 2-hydroxy-4-methyl-, methyl ester3.2140348-72-9146ND0.64ND heptanoic acid, methyl ester3.86000106-73-01440.63ND0.96 butanoic acid, 3-methyl-, 1-methyl3.90032665-23-9144NDND0.11 acetic acid, 2-phenylethyl ester8.13000103-45-71640.380.930.24 bromoacetic acid, 2-phenylethyl ester8.21003785-33-9243NDND0.32 β-phenylethyl butyrate8.28000103-52-6192ND1.680.36 methoxyacetic acid, 2-tetradecyl ester10.691000282-04-8286ND0.460.18 hexanoic acid, 2-ethylhexyl ester11.44016397-75-4228ND0.590.31 hexanoic acid, 3-tetradecyl ester11.581000279-29-7312NDND0.02 pentadecylheptafluorobutanoate11.861000216-79-3424NDND0.03 2-tridecanyl methoxyacetate14.701000282-04-5272NDND0.14 didodecyl phthalate15.43002432-90-8278ND0.060.79 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester15.43000084-69-5502ND0.770.61 1,2-benzenedicarboxylic acid, dipropyl ester15.50000131-16-8250ND0.260.80 hexadecanoic acid, methyl ester15.93000112-39-02700.280.311.80 pentadecanoic acid, 14-methyl-, methyl ester16.13005129-60-2270ND0.500.30 1,2-benzenedicarboxylicacid, 1-butyl 2-(8-methylnonyl) ester16.39000089-18-9362ND0.53ND dibutyl phthalate16.40000084-74-2278ND0.201.72 1,2-benzenedicarboxylic acid, bis(1-methylethyl) ester16.58000605-45-82840.560.961.28 9,12-octadecadienoicacid, methyl ester17.60002462-85-3294NDND0.43 9,12-octadecadienoicacid, methyl ester, (9E,12E)-17.60002566-97-4294NDND0.50 10,13-octadecadienoic acid methyl ester17.60056554-62-2294NDND0.43 linoleic acid ethyl ester18.20000544-35-43081.620.841.83 2-chloropropionic acid, octadecyl ester18.24088104-31-8361NDND0.36 ethyl oleate18.24000111-62-63102.600.631.36 heptadecanoic acid, heptadecyl ester18.92036617-50-2508ND0.300.58 2-propenoic acid, 3-(4-methoxyphenyl)-, 2-ethylhexyl ester19.78005466-77-32900.080.280.80 1-cyclopentene-1-decanoic acid, β-hydroxy-3-oxo-2-pentyl-, methyl ester19.88055521-10-3352ND0.19ND 1,2-benzenedicarboxylic acid, mono(2-ethylhexyl)ester22.32004376-20-92780.150.45ND 1,2-benzenedicarboxylic acid, ditridecyl ester22.32000119-06-2530ND0.460.65 di-n-octyl phthalate22.35000117-84-03900.120.450.29 1,2-benzenedicarboxylic acid, diisooctyl ester22.35027554-26-3390ND0.580.32 ComponentsRT
a/minCAS Registry Number
bM
r
In the present study, a total of 33 esters were detected from three different types of Douchi as shown in Table 6. Most esters have pleasant aromas and enhance Douchi flavor quality
[28]. Acetic acid 2-phenylethyl ester, hexadecanoic acid methyl ester, 1,2-benzenedicarboxylic acid bis(1-methylethyl) ester, linoleic acid ethyl ester, ethyl oleate, 3-(4-methoxyphenyl)-2-propenoic acid 2-ethylhexyl ester and di-n-octyl phthalate were detected in all three samples. Hexadecanoic acid methyl ester, 1,2-benzenedicarboxylic acid bis(1-methylethyl) ester, linoleic acid ethyl ester and ethyl oleate were the major compounds detected. Most of the detected esters were previously found in various fermented soybean foods
[29-31]. A number of high molecular weight fatty acid esters such as pentadecyl heptafluoro butanoate, 1,2-benzenedicarboxylic acid ditridecyl ester and ethyl hexadecanoate were detected, which have also been found in Chinese sufu, miso, and other Korean fermented soybean pastes. These high molecular weight esters were probably produced by the action of fungal lipase on soybean lipids
[32]. The esters concentration of the pure Aspergillus oryzae fermented Douchi was lower than traditionally-fermented and multi-strains fermented Douchi samples, only 6.42 µg/g. The multi-strains fermented Douchi had the highest concentration of esters, i.e. 17.71 µg/g. This may be attributed to the improved Douchi production process and addition of a certain amount of yeast during post-fermentation.
2.7 Heterocyclic compounds
A total of 20 compounds were detected in three different Douchi as shown in Table 7. Pyrazines was the main compound and studies have indicated it was important in fermented soybean products
[33-34], which could be generated naturally during the aging process, by the condensation of aminoketones formed through the Maillard reaction and Strecker degradation. The methylpyrazine, 2,3-dimethylpyrazine, 2,5-dimethylpyrazine, 2,6-dimethylpyrazine, trimethylpyrazine, tetramethylpyrazine and 2-ethyl-6-methylpyrazin have previously been detected in natto and liquid cultures of Bacillus subtilis and sufu, which were also detected in Douchi in the present study. Pyrazines have a nutty aroma, especially alkyl pyrazines. 2,5-Dimethyl pyrazine, trimethylpyrazine and 2-ethyl-6-methyl pyrazine contribute to nattoodour. Tetramethylpyrazine has sweet, chocolate, cocoa, but musty, lard and burnt note. 3-Phenylfuran was detected in traditionally fermented and multistrains fermented Douchi, and this compound was previously found in soybean-processed products such as sufu, miso, soysauce, and natt
[35]. 1-Pentyl-1H-pyrrole was detected in pure Aspergillus oryzae and traditionally fermented Douchi, which has a nutty, sweet and ethereal ordour and may be one of the products of Maillard reaction.
Table7 GC-MS analysis of heterocyclic compounds in three different type types of Douchi

cContent/(μg/g)
dABC Heterocyclic (20)6.5714.0510.35 2-methylpyrazine4.68109-08-0942.081.62ND 2,5-dimethyl pyrazine5.41123-32-0108ND1.183.54 3a,6-methano-3aH-indene, 2,3,6,7-tetrahydro6.4998640-29-0132ND0.07ND 1H-indene, 2,3-dihydro-5-methyl-7.05000874-35-1132ND0.07ND azulene7.18000275-51-4128ND0.270.25 naphthalene7.23000091-20-3128ND0.28ND 1H-indene, 1-methylene-7.30002471-84-3128ND0.09ND 2,3,5-trimethylpyrazine7.4314667-55-11220.591.960.67 2-ethyl-3,5-dimethylpyrazine7.6427043-05-61362.302.582.83 cinnoline, 3-methyl-7.80017372-78-01440.08ND0.10 furan, 3-phenyl-7.92013679-41-9144ND1.960.87 1H-pyrrole, 1-pentyl-9.04000699-22-91370.790.46ND isobutenalmethylphenylhydrazone9.73066400-94-0174ND0.07ND 2,3,5,6-tetramethylpyrazine9.861124-11-41360.650.860.54 4-methyl-6-methoxy-8-aminoquinolin10.91057514-21-3ND0.240.07 1,4-dioxaspiro[4.5]decane, 8-(meth ylthio)-11.44055103-51-00.08NDND 1-(4-bromobutyl)-2-piperidinone13.09195194-80-0234ND0.07ND 2-isopropyl-5-methylpyrazine14.8713925-05-8136ND0.320.45 pyridine, 2-(2-phenylethynyl)-19.88013141-42-9179ND1.650.14 2,4-heptanedione, 6-[[2-(2,3,8,8a-tetrahydro-5,8a-dimethyl-7-oxoimidazo[1,2-a]pyridin-1(7H)-yl)ethyl]imino]-ComponentsRT
a/minCAS Registry Number
bM
r19.88106202-16-8333NDND0.89
2.8 Phenols
Six phenols were identified in three different Douchi as shown in Table 8. 4-Methylphenol and mequinol were found in all three samples, and 2-methoxy-phenol and 2-(1,1-dimethylethyl)-phenol was detected in traditionally and multi-strains fermented Douchi. 2-Methoxy-4-vinylphenol was detected in pure Aspergillus oryzae and multistrains fermented Douchi. 2-Naphthalenol was only detected in traditionally fermented Douchi, most of the above-mentioned phenols were also found in sufu, miso, and soy sauce. Phenols were characterized in cooked soybean with smokey and phenolic odours, and they were considered to be the thermal degradation products of lignin-related phenolic carboxylic acids.
Table8 GC-MS analysis of phenols in three different types of Douchi

Content (μg/g)
dABC Phenols (6)0.452.922.27 4-methylphenol5.43106-44-51080.250.480.32 mequinol5.78000150-76-51240.180.940.70 phenol, 2-methoxy-5.84000090-05-1124ND1.430.65 2-naphthalenol7.80000135-19-3144ND0.05ND 2-methoxy-4-vinylphenol9.32007786-61-01500.02ND0.04 phenol, 2-(1,1-dimethylethyl)-9.32000088-18-6150ND0.020.56 ComponentsRT
a/minCAS Registry number
bM
r
c
2.9 Other compound
A total of 9 compounds were detected in three different Douchi as shown in Table 9 including 3 sulfur-containing compounds. These compounds had a significant contribution to the aroma in foods (Fors, 1983). 5,6-Dihydro-2H-thiopyran and di-tert-dodecyl disulfide were detected in traditionally and multi-strains fermented Douchi. Dimethyl trisulfide was detected in pure Aspergillus oryzae and traditional fermented Douchi. It is one of the major flavor components in natto. 9-Octadecenamide and 13-docosenamide were both found in traditional fermented Douchi, which were also detected in oatmeal
[36].
Table9 GC-MS analysis of other compounds in three different types of Douchi
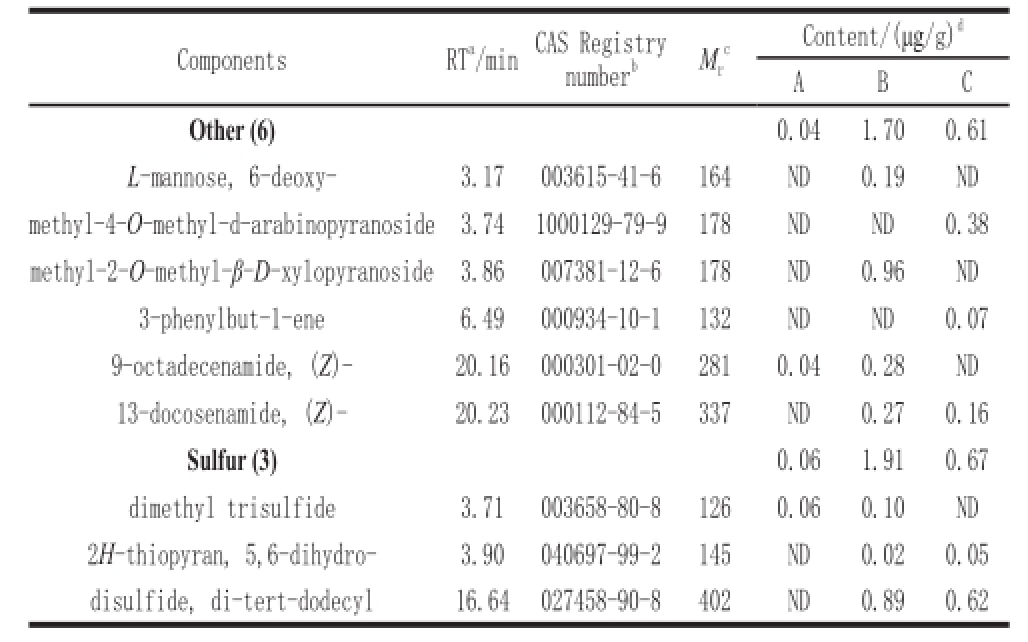
Content/(μg/g)
dABC Other (6)0.041.700.61 L-mannose, 6-deoxy-3.17003615-41-6164ND0.19ND methyl-4-O-methyl-d-arabinopyranoside3.741000129-79-9178NDND0.38 methyl-2-O-methyl-β-D-xylopyranoside3.86007381-12-6178ND0.96ND 3-phenylbut-1-ene6.49000934-10-1132NDND0.07 9-octadecenamide, (Z)-20.16000301-02-02810.040.28ND 13-docosenamide, (Z)-20.23000112-84-5337ND0.270.16 Sulfur (3)0.061.910.67 dimethyl trisulfide3.71003658-80-81260.060.10ND 2H-thiopyran, 5,6-dihydro-3.90040697-99-2145ND0.020.05 disulfide, di-tert-dodecyl16.64027458-90-8402ND0.890.62 ComponentsRT
a/minCAS Registry number
bM
r
c
From the results obtained, the concentrations of volatile compounds were various along with different Douchi samples. The situation were feasibly due to variety of microorganisms and some variations in the proprietary processing steps which used by each manufacturer. Pyrazines were identified in soybean-processed products such as sufu, miso, soy sauce, and natto previously, this class of compound together with other common compounds probably contributes more to the characteristic background flavour of all samples investigated. Less predominant groups including esters, acids, alcohols, and aldehydes might play an indispensable role in the flavour of Douchi.
The comparison of the results showed that the pure Aspergillus oryzae fermented sample had higher hydrocarbons, aldehydes and acids than traditionally fermented Douchi, while these compounds are not the important proups. Therefore, the odor of pure Aspergillus oryzae fermented sample is not as good as the traditionally fermented sample. On the other hand, multi-strains fermented Douchi has higher alcohols, esters andpyrazines than the pure Aspergillus oryzae fermented sample. And these compounds are the major aroma active compounds, so the odor of multi-strains fermented Douchi is better than the pure Aspergillus oryzae fermented sample.
3 Conclusion
In three different types Douchi samples, a total of 152 combined volatile compounds were identified. Ten classes of compounds including hydrocarbons, alcohols, aldehydes, ketones, acids, esters, pyrazines, phenols, mis-cellaneousand sulfur-containing compound were detected. The traditional fermented samples had higher contents and classes than the Aspergillus oryzae fermented and multi-strains fermented samples. In order to improve the odor of Aspergillus oryzae fermented Douchi, further research is required on the development of aroma active compound during the fermentation of Douchi and optimize the raw materials, microbial community and fermentation conditions worth more attention.
References:nces
[1] LUO Yangchao, LI Bo, HONG Ji, et al. Effect of soaking and cooking on selected soybean variety for preparation of fibrinolytic Douchi[J]. Journal of Food Science and Technology-Mysore, 2008, 46(2): 104-108.
[2] LUO Yangchao, LI Bo, HONG Ji, et al. Effect of soybean varieties on the fibrinolytic activity and sensory characteristics of Douchi[J]. Journal of Food Processing and Preservation, 2010, 34(Suppl 2): 457-469. DOI:10.1111/j.1745-4549.2008.00297.x.
[3] KATEKAN D, ARUNEE A, EKACHAI C. Volatile profiles of thua nao, a Thai fermented soy product[J]. Food Chemistry, 2011, 125(2):464-470. DOI:10.1016/j.foodchem.2010.09.030.
[4] ZHAO Jianxin, GU Xiaohong, LIU Yangmin, et al. Study on the volatile flavor compounds of the traditional Chinese soybean paste[J]. Food Science, 2006, 27(12): 684-687. DOI:10.3321/ j.issn:1002-6630.2006.12.177.
[5] ETSUKO S, TETSUO I, SATOSHI O, et al. Comparison of compositions of odor components of natto and cookeds oybeans[J]. Agricultural and Biological Chemistry, 1985, 49(2): 311-317. DOI:10. 1080/00021369.1985.10866740.
[6] TADAYOSHI T, KANAKO M, HAENG R K, et al. Comparison of volatile compounds from chungkuk-jang anditohiki-natto[J]. Bioscience, Biotechnology, and Biochemistry, 1998, 62(7): 1440-1444. DOI:10.1271/bbb.62.1440.
[7] OUOBA LI, DIAWARA B, ANNAN N T, et al. Volatile compounds of Soumbala, a fermented African locust bean (Parkia biglobosa) food condiment[J]. Journal of Applied Microbiology, 2005, 99(6): 1413-1421. DOI:10.1111/j.1365-2672.2005.02722.x.
[8] LEE S J, BOMI A. Comparison of volatile components in fermented soybean pastes using simultaneous distillation and extraction (SDE)with sensory characterisation[J]. Food Chemistry, 2009, 114(2): 600-609. DOI:10.1016/j.foodchem.2008.09.091.
[9] WANG Lijun, MU Huiling, LIU Haijie et al. Volatile components in three commercial Douchies, a Chinese traditional salt-fermented soybean Food[J]. International Journal of Food Properties, 2010, 13(5): 1117-1133. DOI:10.1080/10942910902968726.
[10] QIN Likang, DING Xiaolin. Formation of taste and odor compounds during prepartion of Douchiba, A Chinese traditional soy-fermented appetizer[J]. Journal of Food Biochemistry, 2007, 31(2): 230-251. DOI:10.1111/j.1745-4514.2007.00105.x.
[11] ZHANG Yanfang, TAO Wenyi. Flavor and taste compounds analysis in Chinese solid fermented soy sauce[J]. African Journal of Biotechnology, 2009, 26(17): 210-216.
[12] ZHENG Wencheng, GUAN Bo. Extraction and purification of squalene and its application[J]. Science and Technology of Cereals, Oils and Foods, 2010, 18(4): 27-30. DOI:10.16210/ j.cnki.1007-7561.2010.04.001.
[13] STEINHAUS M, SINUCO D, POLSTER J, et al. Characterization of the key aroma compounds in pink guava (Psidium guajava L.) by means of aroma re-engineering experiments and omission tests[J]. Journal of Agricultural and Food Chemistry, 2009, 57(7): 2882-2888. DOI:10.1021/jf803728n.
[14] MYSORE N S, REVATHY B, LINGAMALLU J R, et al. Influence of processing conditions on flavour compounds of custard apple (Annona squamosa L.)[J]. LWT-Food Science and Technology, 2008, 41(2):236-243. DOI:10.1016/j.lwt.2007.03.005.
[15] CHIOU R Y, FERNGS, BEUCHAT L R. Fermentation of low-salt miso as affected by supplementation with ethanol[J]. International Journal of Food Microbiology, 1999, 48(1): 11-20. DOI:10.1016/ S0168-1605(99)00033-1.
[16] LI Qin, DU Fenggang. Study on Improvement of soy sauce flavor by adding fragrance-producing yeast during fermentation[J]. China Brewing, 2003, 21(16): 27-28.
[17] CHUNG H Y. Volmpoatile conents in fermented soybean (Glycine max) curds[J]. Journal of Agricultural and Food Chemistry, 1999, 47(7): 2690-2696. DOI:10.1021/jf981166a.
[18] CHUNG H Y. Volatile flavor components in red fermented soybean(Glycinemax) curds[J]. Journal of Agricultural and Food Chemistry, 2000, 48(5): 1803-1809. DOI:10.1021/jf991272s.
[19] KU K L, CHEN T P, CHIOU R Y. Apparatus used for small-scale volatile extraction from ethanol-supplemented low-salt miso and GCMS characterization of the extracted flavors[J]. Journal of Agricultural and Food Chemistry, 2000, 48(8): 3507-3511. DOI:10.1021/ jf9910483.
[20] ETSUKO S, YUICHI Y K. Comparison of aroma componets in five types of miso[J]. Nippon Shokuhin Kagaku Kogaku Kaishi, 1998, 45(5): 323-329. DOI:10.6013/jbrewsocjapan1988.94.435.
[21] ARUNSRI L, CRAIG D S, DAVID O J, et al. Volatile compounds in Bacillus-fermented soybeans[J]. Journal of the Science of Food and Agriculture, 2001, 81(5): 525-529. DOI:10.1002/jsfa.843.
[22] PITIPONG W, SITTIWAT L. Comparison of determination method for volatile compounds in Thai soy sauce[J]. Food Chemistry, 2003, 83(4): 619-629. DOI:10.1016/S0308-8146(03)00256-5.
[23] JENNIFER M A, GLESNI M. Volatile components of an unflavored textured soy protein[J]. Journal of Food Science, 1984, 49(6):1552-1565. DOI:10.1111/j.1365-2621.1984.tb12842.x.
[24] DAVID O J, NANCY A, GARY K, et al. Formation of volatile compounds during bacillus subtilis fermentation of soya beans[J]. Journal of the Science of Food and Agriculture, 1997, 74(1):132-140. DOI:10.1002/(SICI)1097-0010(199705)74:1<132::AIDJSFA779>3.0.CO;2-8.
[25] LIU Zhencheng. The study on volatile flavor compounds of Chinese traditional soy sauce[J]. Master Degree Dissertation of South China University of Technology, 2012, 35(16): 102-107.
[26] CHUNG H Y, FUNG P K, KIM J S. Aroma impact components in commercial plain sufu[J]. Journal of Agricultural and Food Chemistry, 2005, 53(5): 1684-1691. DOI:10.1021/jf048617d.
[27] MIREILLE Y, LIESBETH R. Cheese flavour formation by amino acid catabolism[J]. International Dairy Journal, 2001, 11(4/5/6/7): 185-201. DOI:10.1016/S0958-6946(01)00049-8.
[28] KWANG J J, MYO R S. Flavor components generated from thermally processed soybean paste (Doenjang and Soondoenjang) soups and characteristics of sensory evaluation[J]. Journal of Physics and Chemistry of Solids, 2004, 36(2): 175-186.
[29] PARK H K, BOGIM G, JONG K K. Characteristic flavor compounds of commercial soybean paste[J]. Food Science and Biotechnology, 2003, 12(6): 607-611.
[30] PARK J S, LEE M, KIM K S, et al. Volatile flavor components of soybean paste(Doenjang) prepared from different types of strains[J]. Journal of Classroom Interaction, 2014, 49(12): 26-32.
[31] JOO K J, SHIN M R. Fractionated volatile flavor components of soybean paste by dynamic headspace method[J]. Journal of Korean Society of Food Science and Nutrition, 1999, 55(23): 76-81.
[32] CHOU C C, HWAN C H. Effect of ethanol on the hydrolysis of protein and lipid during the ageing of a Chinese fermented soya bean curd- Sufu[J]. Journal of the Science of Food and Agriculture, 1994, 66(3): 393-398. DOI:10.1002/jsfa.2740660318.
[33] YUTAKA M, KAN K, HIDEO T. Flavor components of miso:basic fraction[J]. Agricultural and Biological Chemistry, 1983, 47(7): 1487-1492.
[34] SEO J, CHANG H, JI W, et al. Aroma components of traditional Korean soy sauce and soybean paste fermented with the same Meju[J]. Journal of Microbiology and Biotechnology, 1996, 35(16): 278-285.
[35] ETSUKO S. Change in aroma components of miso with aging[J]. Nippon Shokuhin Kogyo Gakkaishi, 1991, 38(12): 1093-1097. DOI:10.3136/nskkk1962.38.1093.
[36] SUN Peipei, HUANG Mingquan, SUN Baoguo, et al. Study on volatile components in oats flakes by simultaneous distillation extraction and gas chromatography-mass spectrometry[J]. Science and Technology of Food Industry, 2011, 32(12): 479-483.
传统霉菌发酵与接种发酵豆豉风味物质的比较分析
叶 艳,苏 伟*,王 倩,何 欣,高静雅
(江西科技师范大学生命科学学院,江西 南昌 330013)
摘 要:对接种发酵制作的豆豉香气成分进行分析检测,并与传统自然发酵豆豉和纯种米曲霉发酵的豆豉进行比较,经GC-MS分析,一共鉴定出了烃类、醇类、醛类、酮类、酸类、酯类、杂环化合物、含硫化合物、酚类以及其他化合物共10 类,152 种挥发性成分;结果表明,混合菌种发酵豆豉所含的烃类、醛类和酸类物质均高于纯种发酵培养,而传统自然发酵产生的醇类、杂环化合物、含硫化合物、酚类以及其他化合物都高于纯种发酵及混合菌种豆豉,而混合菌种发酵豆豉在酮类和酯类化合物要明显高于纯种米曲霉豆豉和传统自然发酵豆豉。
关键词:豆豉;发酵;风味物质
中图分类号:TS26
文献标志码:A
文章编号:1 0 0 2-6 6 3 0(2 0 1 6)2 0-0 0 8 6-0 9
引文格式:
收稿日期:2016-04-24
基金项目:江西科技师范大学本科生创业、科研基金项目(20150904027)
作者简介:叶艳(1993—),女,本科生,研究方向为食品生物技术。E-mail:oooyyyee@163.com
*通信作者:苏伟(1971—),女,副教授,硕士,研究方向为食品生物技术。E-mail:suwei74@hotmail.com
DOI:10.7506/spkx1002-6630-201620015








