LI Kun 1,2, ZHENG Hua 1,2, ZHANG Wenwen 1, LI Kai 1, XU Juan 1, ZHANG Hong 1,2,*
(1. Research Institute of Resources Insects, Research Center of Engineering and Technology on Forest Resources with Characteristics, State Forestry Administration, Chinese Academy of Forestry, Kunming 650224, China; 2. Key Laboratory of Cultivation and Utilization of Resource Insects, State Forestry Administration, Kunming 650224, China)
Abstract:For the purpose of avoiding the risks arising from the application of traditional bleached shellac containing bound chlorine in the fields such as food and medicine, a totally chlorine-free (TCF) bleaching technique for shellac was developed to extend the scope of application of bleached shellac in these fields. Response surface methodology (RSM) was employed to research the influences of process parameters including ratio of shellac to bleaching solution, hydrogen peroxide (H 2O 2) dosage, bleaching temperature, bleaching time, stabilizer dosage on bleaching efficiency. As a result, the optimum levels of these parameters were determined as 0.08 g/mL, 2.09 mL/g (on the basis of shellac), 80 ℃, 7 h, and 3.125% (on the basis of shellac), respectively. Further, the obtained H 2O 2bleached shellac was compared with NaClO bleached shellac in term of quality indicators, Fourier transform infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC) characteristics. Results showed that the molecular structure of the H 2O 2bleached shellac was basically consistent with that of the NaClO bleached shellac and the alkali extracted shellac. Moreover, cold ethanol soluble substance, hot ethanol insoluble substance, thermal lifetime of the H 2O 2bleached shellac were improved greatly. But the acid value was increased and the softening point was decreased significantly. The color index was 0.979 (less than 1.0) and the yield of bleached shellac was 82.03%. It was shown that TCF bleached shellac was an excellent additive and raw material for the food industry.
Key words:shellac; totally chlorine-free bleaching; process; modeling and optimization
LI Kun, ZHENG Hua, ZHANG Wenwen, et al. Modeling and optimization of totally chlorine-free bleaching technique of shellac using response surface methodology and characterization of H 2O 2bleached shellac[J]. 食品科学, 2016, 37(22): 41-51. DOI:10.7506/spkx1002-6630-201622007. http://www.spkx.net.cn
LI Kun, ZHENG Hua, ZHANG Wenwen, et al. Modeling and optimization of totally chlorine-free bleaching technique of shellac using response surface methodology and characterization of H 2O 2bleached shellac[J]. Food Science, 2016, 37(22): 41-51. DOI:10.7506/spkx1002-6630-201622007. http://www.spkx.net.cn
Lac, also called insect lac or red lac, is an amaranthine natural resin secreted by lac insect living in a host plant [1-2]and it is named as ‘shellac’ in traditional Chinese medicine [3]. Shellac is a typical kind of thermoplastic resin characterized by good wear and oil resistance, salt spray and salinityproofing ability, preferable bonding and film forming properties [4-5]. Thereby, it has been widely used in military, electronic and coating industry at its initial time [6-8]. In the recent years, with the constant focus on food and medicine, shellac has been widely applied due to its green, nontoxic and natural properties. The application scope refer to food includes: anti-blushing agent for food, curing agent, coating agents for fruit refreshing [9-13]. Regarding its roles in medicine, it involves the tablets with sustained release and targeting effect, coating of capsule in pharmaceutical industry [14-18], adhesive film for dental treatment and oral administration composition etc. [19-21]. In fact, as the production of sticklac has been declined rapidly after World War Ⅱ, its price has been sharply increased recently. The application of shellac, especially of bleached shellac, has been an irreversible trend in the industries with high added value, such as in food, medicine and cosmetic industry.
At present, traditional and extensive sodium hypochlorite bleaching technique still plays a main role on shellac bleaching in China. However, the usage of bleached shellac in food, medicine, cosmetic industries was gravely restricted because of the existence of numerous combined chlorine [22]. And the storage period of products of bleached shellac was shorten in 6 months [23]. Therefore, the marketability of bleached shellac was reduced greatly and its application range was limited [24]. In the study, a complete H 2O 2bleaching technique of shellac was developed to solve the problem, the existence of many combined chlorine in bleached shellac of sodium hypochlorite bleaching technique. In addition, totally chlorine-free (TCF) bleached shellac was realized with green and nontoxic bleaching process. The study provides raw materials to expand application areas of bleached shellac and also provides reference and revelation for further development of bleaching technique of shellac, especially for TCF bleaching technique.
1.1 Materials and instruments
Seedlac and sodium hypochlorite bleached shellac were provided by Kunming Xilaike Bio-technology Co. Ltd. (Kunming, China). Sodium silicate, magnesium sulfate, hydrogen peroxide, sodium carbonate and potassium hydroxide were all analytical grade and purchased by Tianjin Zhiyuan Chemical Reagent Co. Ltd. (Tianjin, China).
DF101-S Thermostatic magnetic stirrer was obtained from Zhengzhou Nanbei Instrument Equipment Co. Ltd., China; CPC-505 conductivity meter was obtained from Smart Instrument Company (Germany); HR83 halogen moisture analyzer was purchased from Switzerland Mettler Toledo Co. Ltd., (China); BP2215 electronic balance was obtained from Sartorius Corporation (Germany); FD-1C-50 vacuum freeze dryer was obtained from Beijing Boyikang Experiment Instrument Co. Ltd., (China); TY742X2A pure water machine was purchased from Thermo Fisher Barnstead Corporation (USA); DSC200F3 differential scanning calorimeter (DSC) was purchased from Nestch Corporation (Germany); High purity nitrogen (purity is more than 99.999%) and liquid nitrogen were obtained from Kunming Messer Gas Products Co. Ltd. (Kunming, China); Tensor-27 Fourier transform infrared (FTIR) spectrometer were obtained from Bruker Corporation (Germany); 904 Titrando electric titrator was purchased from Metrohm Corporation, (Switzerland).
1.2 Methods
1.2.1 Preparation of raw shellac for bleaching
Four hundred grams of seed lac was weighed up and dissolved into 4 liter of solution with 0.1 mol/L of Na 2CO 3at 50 ℃. After cooling down at room temperature, it was put into refrigerator at 4 ℃ for 24 h. Then some filter liquor was acquired after suction filtration. And solid sodium chloride was added for salting out. After suction filtration, a
certain filter cake was taken. Then raw shellac for bleaching (hereinafter referred to as alkali extracted shellac for convenience) was obtained after vacuum drying at 40 ℃.
1.2.2 Determination method of the color index of bleached shellac
The qualitative indexes including color index, hot ethanol insoluble substance, cold ethanol insoluble substance, thermal lifetime, softening point, and acid value of bleaching shellac were measured based on shellac product inspection method of Chinese national standard, GB/T 8143—2008 Test methods of lac products [25].
1.2.3 Computing method of the yield of bleached shellac
The calculation formula for the yield of bleached shellac is:
Where T is the yield of bleached shellac/%; M 0is the mass of weighed alkali extracted shellac/g; M 1is the mass of bleached shellac after bleaching/g; A 0is the moisture content of alkali extracted shellac/%; and A 1is the moisture content of bleached shellac/%.
1.2.4 Single factor experiment for bleaching technique of shellac
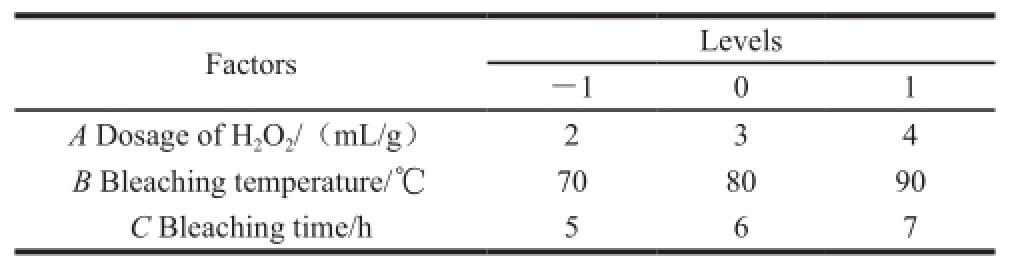
1.2.4.1 Influence of the concentration of shellac in bleaching liquor
Na 2CO 3solution of 0.15 mol/L prepared with 3.18 g of Na 2CO 3and 200 mL of water were put into a boiling flask-3-neck (500 mL). Then raw shellac prepared in section 1.2.1 with 10, 12, 14, 16, 18, 20 and 22 g were added respectively. The shellac bleaching liquors with mass concentrations of 0.05, 0.06, 0.07, 0.08, 0.09, 0.10 g/mL and 0.11 g/mL were prepared after dissolution by heating. And 0.5 g of Na 2SiO 3and 0.125 g of MgSO 4were added. Then H 2O 2was dropped into the solution with a ratio to shellac of 3:1 (mL/g). The liquors were bleached for 5 hours at 85 ℃. After suction filtration and cooling, 5% H 2SO 4solution was dropped for acidification. Then bleached shellac was obtained after filtration. After washing and freeze-drying, the yield and color index of bleached shellac were acquired using the methods described in sections 1.2.2 and 1.2.3.
1.2.4.2 Influence of the dosage of H 2O 2
Firstly, 200 mL of water and Na 2CO 3solution of 0.15 mol/L prepared with 3.18 g of Na 2CO 3were put into a boiling flask-3-neck of 500 mL respectively, 16 g of raw shellac was prepared in 1.2.1 as well. After dissolution by heating, 0.5 g of Na 2SiO 3and 0.125 g of MgSO 4were added, respectively. Then H 2O 2was dropped in different proportions to shellac, namely, 1, 2, 3, 4, and 5 mL/g. They were bleached for 5 hours at 85 ℃. After suction filtration and cooling, 5% H 2SO 4solution was dropped to acidize. Then bleached shellac was acquired by filtering. After washing and freeze-drying, the yield and color index of bleached shellac were obtained by the same methods in 1.2.4.1.
1.2.4.3 Influence of bleaching temperature
The dosage of H 2O 2was ensured according to the result obtained from 1.2.4.2. After adding 0.5 g of Na 2SiO 3and 0.125 g of MgSO 4, H 2O 2was dropped in proportions to shellac of 2 mL/g. Then bleaching was preformed at 50, 60, 70, 80 and 90 ℃, respectively, for 5 hours. Afterwards the yield and color index of bleached shellac were obtained using the same steps in 1.2.4.1.
1.2.4.4 Influence of bleaching time
After the same first step as that of 1.2.4.2 and adding the same amounts of Na 2SiO 3and MgSO 4in the same way, H 2O 2was dropped according to the ratio of 3:1 (mL/g) between H 2O 2and shellac. At the bleaching temperature of 80 ℃, it was bleached from 1 to 7 hours respectively. Then the same steps in 1.2.4.1 were utilized to obtain the yield and color index of bleached shellac.
1.2.4.5 Influence of the dosage of stabilizer
The first step was still same but H 2O 2was dropped in a proportion of 3 mL/g between H 2O 2and shellac. Then Na 2SiO 3and MgSO 4were added in mass ratios of 0.5:0.125, 1:0.25, 1.5:0.375, 2:0.5, 2.5:0.625, 3:0.75, 3.5:0.875 and 4:1, respectively. In order to express conveniently, hereinafter the mass percentage of Na 2SiO 3in shellac was employed. It suggested that the dosages of stabilizer were 3.125%, 6.25%, 9.375%, 12.5%, 15.625%, 18.75%, 21.875% and 25%, respectively. Then it was bleached for 6 hours at 80 ℃. After that, the yield and color index of bleached shellac were calculated in the same way as that in 1.2.4.1.
1.2.5 Optimization experiment design of bleaching technique of shellac
Based on the results of single factor experiments in 1.2.4, the dosage of H 2O 2, bleaching temperature and time were taken as the response variables, the yield of bleached shellac was calculated according to its mass after drying and the color index was computed by the method of GB/T 8143—2008 as well. With the yield and color index of bleached shellac as response values, an experiment design based on Box-Behnken composite theory was conducted using Design-Expert 8.0.6 Software. The experiment factor level was presented in Table1. Then on the basis of the results, an optimization analysis was carried out to obtain the optimum conditions.
Table1 The coded values and corresponding actual values of the independent variables used in the response surface analysis
1.2.6 Comparative analysis of H 2O 2and sodium hypochlorite bleached shellac
1.2.6.1 Contrastive analysis of qualitative indexes for both bleached shellac
Seed lac, alkali extracted shellac, the bleached shellac obtained by traditional sodium hypochlorite bleaching technique and H 2O 2bleached shellac were determined by the method of shellac product inspection in GB/T 8143—2008, the indexes, including hot ethanol insoluble substance, cold ethanol soluble substance, softening points, thermal lifetime, color index, acid value, were measured respectively and the results were analyzed comparatively.
1.2.6.2 FTIR analysis of both bleached shellac
Tensor-27 FTIR spectrometer was applied to conduct infrared spectroscopic analysis of the shellacs mentioned with the following test conditions, i.e., scanning range was 4 000-400 cm -1, resolution was 4 cm -1and cumulative scanning was 32 times.
1.2.6.3 DSC analysis of both bleached shellac
Heating-up scanning of 5 mg of each lac above from 0 to 150 ℃ with a rate of 10 ℃/min was carried out by DSC. In order to ensure complete fusion of all samples and steady base line, after keeping constant temperature for 2 minutes, cooling scanning was performed from 150 to 0 ℃ with a rate of 10 ℃/min. The similarities and differences of thermal properties of both bleached shellac and the differences in composition and property with seed lac were contrastively analyzed.
2.1 Single factor experiment results
2.1.1 Influence of the concentration of shellac solution on bleaching effect
The concentration of shellac bleaching liquor should be fixed firstly during bleaching, because it affects the dosage of H 2O 2, even some relevant bleaching conditions. Fig.1 indicates that the yield of bleached shellac was increased greatly with increasing concentration of bleaching liquor. The sudden change in color index appeared when the concentration of bleaching liquor was 0.08 g/mL. When the concentration was lower than 0.08 g/mL, the color index was changed slowly. When it was higher than 0.08 g/mL, the color index was increased rapidly. Thereby, within the variation range of the concentration of shellac, 0.08 g/mL was the optimal experimental condition chosen for further optimization.
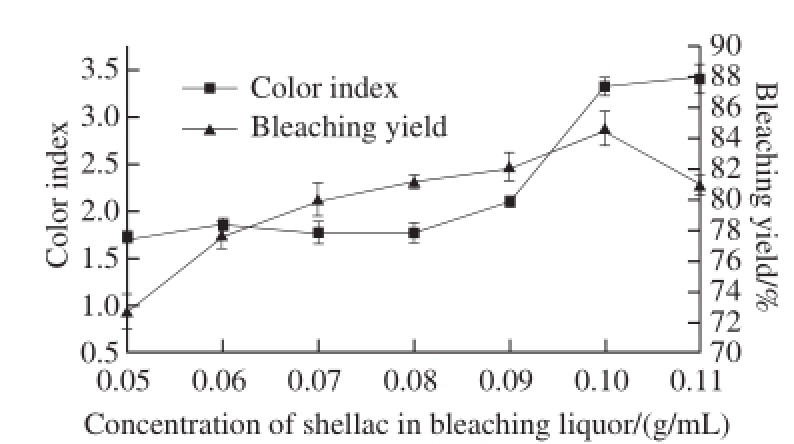
Fig.1 Effect of ratio of shellac to bleaching solution on the color index and yield of bleached shellac
2.1.2 The influence of the dosage of H 2O 2on bleaching effect
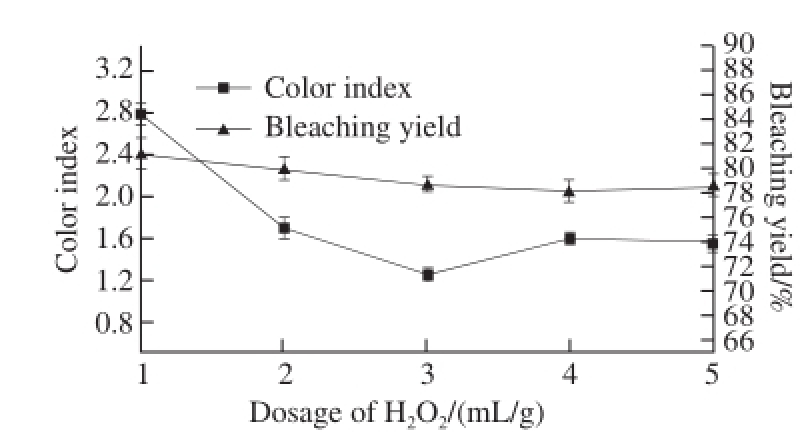
Fig.2 Effect of H 2O 2dosage on the color index and yield of bleached shellac
It is obvious that with the increasing dosage of H 2O 2, the color index of bleached shellac reduced rapidly from Fig.2. When the dosage of H 2O 2was higher than 2 mL/g, all color indexes were below 2.0, which was the minimum standard of bleached shellac according to GB/T 8140—2009. When the dosage of H 2O 2was 3 mL/g, the color index was minimized to 1.57. In view of the yield of bleached shellac, it tended to show a downward trend in the bleaching process but it was changed slightly for the whole. Therefore, the dosage of H 2O 2with 3 mL/g as the optimization experiment center was considered to be optimal.
2.1.3 The influence of bleaching temperature on bleaching effect
As shown in Fig.3, the color index of bleached shellac was decreased along with rising bleaching temperature. The color index was 8.10 at 50 ℃ but it was reduced rapidly to 0.44 at 90 ℃. When the bleaching temperature was higher than 70 ℃, the color index was decreased sharply from 5.84
at 70 ℃ to 1.11 at 80 ℃ suggesting that the bleaching activity of H 2O 2was improved greatly when the temperature was higher than 70 ℃. The decreasing trend of yield of bleached shellac with increasing bleaching temperature was consistent with the change of the color index whereas the yield of bleached shellac was changed suddenly at 80 ℃ and was reduced from 91.44% to 83.02% at 90 ℃. Through systemic study on the variation features of these two response values, it can be known that 80 ℃ is the optimal bleaching temperature as the center point of the optimization experiment.

Fig.3 Effect of bleaching temperature on the color index and yield of bleached shellac
2.1.4 The influence of bleaching time on bleaching effect
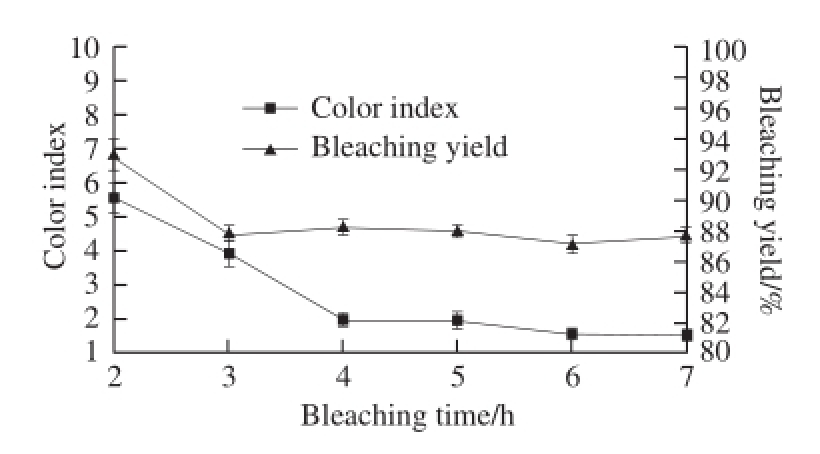
Fig.4 Effect of bleaching time on the color index and yield of bleached shellac
With the extension of bleaching time, the color index of bleached shellac was declined significantly and the reduction in the first four hours was greater, while it was slowed down after the 4 thhour (see Fig.4). It illustrates that the bleaching reaction between H 2O 2and coloring matters in shellac mainly happened in the first four hours. And after the 6 thhour, the color index of bleached shellac was 1.57, which was less than 2.0, and met the requirements of ordinary bleached shellac Grade Ⅱ according to China national standard of GB/T 8140—2009 Bleached shellac [26]. In addition, the changing curve of the yield of bleached shellac showed that the yield was reduced rapidly in the first 3 hours, but it leveled off later. Hence, considering the changes in the color index and yield, 6 h is the optimal bleaching time for conducting the optimization experiment.
2.1.5 The influence of the dosage of stabilizer on bleaching effect

Fig.5 Effect of stabilizer dosage on the color index and yield of bleached shellac
The change of the color index shown in Fig.5 presented that the application of stabilizer greatly affected the color index of bleached shellac. When the mass of stabilizer just accounted for 3.125% of shellac, the color index of bleached shellac was reduced from 12.28 to 2.01, while when the dosage of stabilizer was increased to 25%, the color index was 1.08, which was just decreased by 0.93 (less than 1.0). It suggests that with or without stabilizer had a great influence on the color index of bleached shellac, while considering the conditions in the paper, the influence of the level of stabilizer use on bleaching effect was insignificant. However, as indicated by the changing curve of the yield of bleached shellac, the yield of bleached shellac was decreased to some extent with the continuously increasing dosage of stabilizer. Taken all the factors into consideration,, the dosage of stabilizer with 3.125% can be suitable. In fact, it is important to point out that the level of stabilizer use is not directly related to the color index of bleached shellac. Actually, it influenced the stabilization and survival time of H 2O 2to affect the bleaching effect of shellac. Apparently, there is no need to take the dosage of stabilizer as an optimizing factor of bleaching technique, but it should be within an appropriate range.
2.2 Optimization experiment of bleaching technique of shellac by response surface method
The dosage of H 2O 2, bleaching temperature and bleaching time were applied as response variables and the yield of bleached shellac was employed as response value (Y 1). Then Design-Expert 8.0.6 Software was utilized for cubic polynomial fitting by nonlinear regression and the equation obtained was:
Y 1=76.64-5.89A-9B-1.73C-1.59A 2+2.32B 2+1.37C 2+0.25AB-2.92AC-0.087BC+1.08A 2B+2.32AB 2
Table2 Box-Behnken experimental design with experimental results for the responses
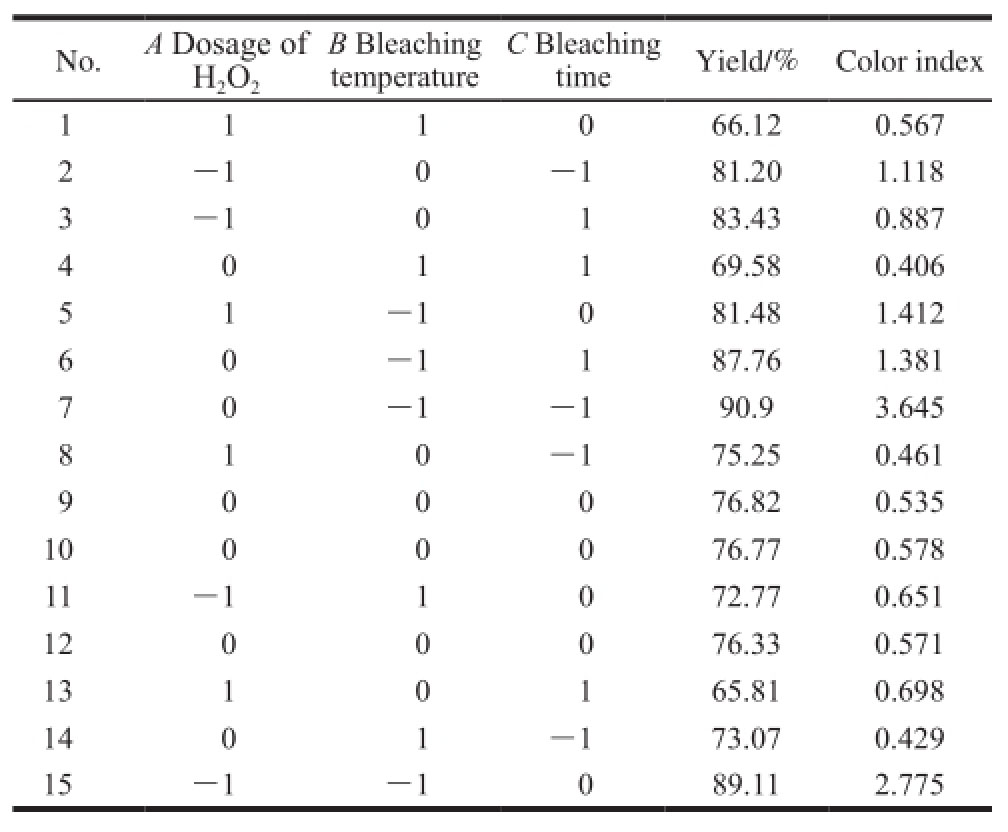
Table3 Analysis of variance of regression equation with the yield of bleached shellac as response variable

Note: P<0.05, Significant influence. The same apply to Table4.
Table3 demonstrates that based on P value, the influences of all linear terms on the yield of bleached shellac were significant, especially the effect of the dosage of H
2O
2and bleaching temperature. And among interaction terms, only the combination of the dosage of H
2O
2and bleaching time reached a significant level, while all quadratic terms had significant effects on the yield of bleached shellac and the most influencing factor was bleaching temperature, followed by the dosage of H
2O
2and bleaching time. The impact of two correction terms on the yield of bleached shellac reached a significant level as well. It means that quadratic polynomial fitting between the yield of bleached shellac and each influence factor was not perfect. Thus, the model was needed to be modified. After modification, the model had conspicuous response to the changes in all the influencing factors (P value of model was less than 0.000 1), and the P value of lack of fit was 0.526 4, which was more than 0.05. Besides, the regression coefficient of model was R
2=0.999 8 and the adjusted regression coefficient after modification was
![]() Both were more than 0.999. Above all, the fitting degree of model equation was good.
Both were more than 0.999. Above all, the fitting degree of model equation was good.
The response surfaces and contour diagrams based on the regression equation mentioned were illustrated in Fig.6.
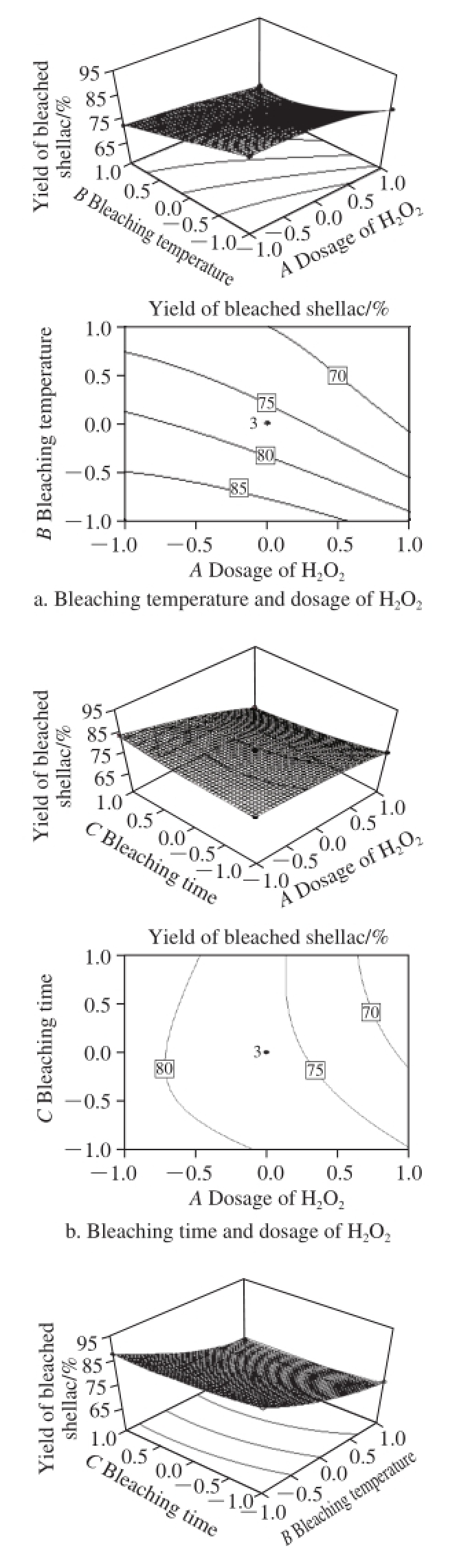
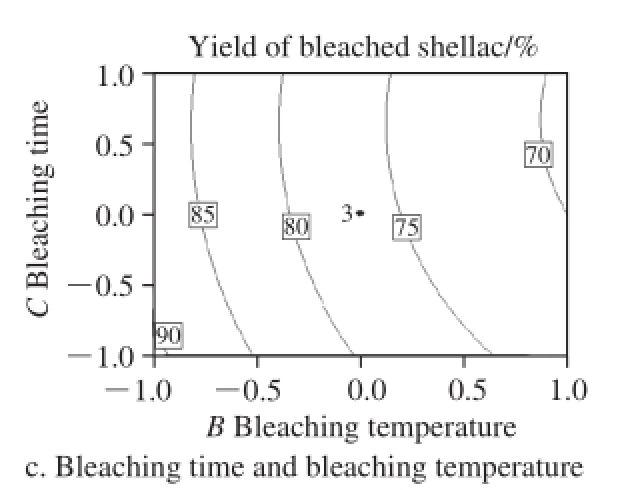
Fig.6 Response surfaces and contours showing the effects of process conditions on the yield of bleached shellac
In Fig.6a, the yield of bleached shellac was declined obviously. Thus, high temperature and dosage of H 2O 2accelerated the degradation reaction of shellac in bleaching. However, because H 2O 2was non-selective for bleaching of coloring matter under the current system, the degradation or oxidation of shellac resin by bleaching agent was the ineviTableside effect during bleaching. Therefore, both the yield of bleached shellac and the change of the color index should be chosen as bleaching conditions.
It is easy to see that with the increase in the dosage of H 2O 2and bleaching time, the yield of bleached shellac was reduced greatly from Fig.6b. In order to enhance the yield, the reaction time between H 2O 2and bleached shellac should be minimized after the color index met the requirements to decrease the degradation of shellac by H 2O 2.
From Fig.6c, it is obvious that within the optimization range selected by the model, the effect of bleaching temperature was greater than that of bleaching time on the yield of bleached shellac. Besides, the higher the bleaching temperature was, the lower the yield.
To sum up, the yield of bleached shellac was impacted greatly by the dosage of H 2O 2, bleaching temperature and bleaching time. The conditions of the model affecting the yield of bleached shellac were in a descending order as follows: bleaching temperature > the dosage of H 2O 2> bleaching time.
Regarding the dosage of H 2O 2, bleaching temperature and bleaching time as response variables and the color index as response value (Y 2), the equation was obtained using Design-Expert 8.0.6 Software for quadratic polynomial fitting by nonlinear regression as follows:
Y 2=0.75-0.12A-0.45B-0.19C+0.074A 2+0.28B 2+0.061C 2+ 0.1AB+0.065AC+0.18BC+0.13A 2B+0.19A 2C
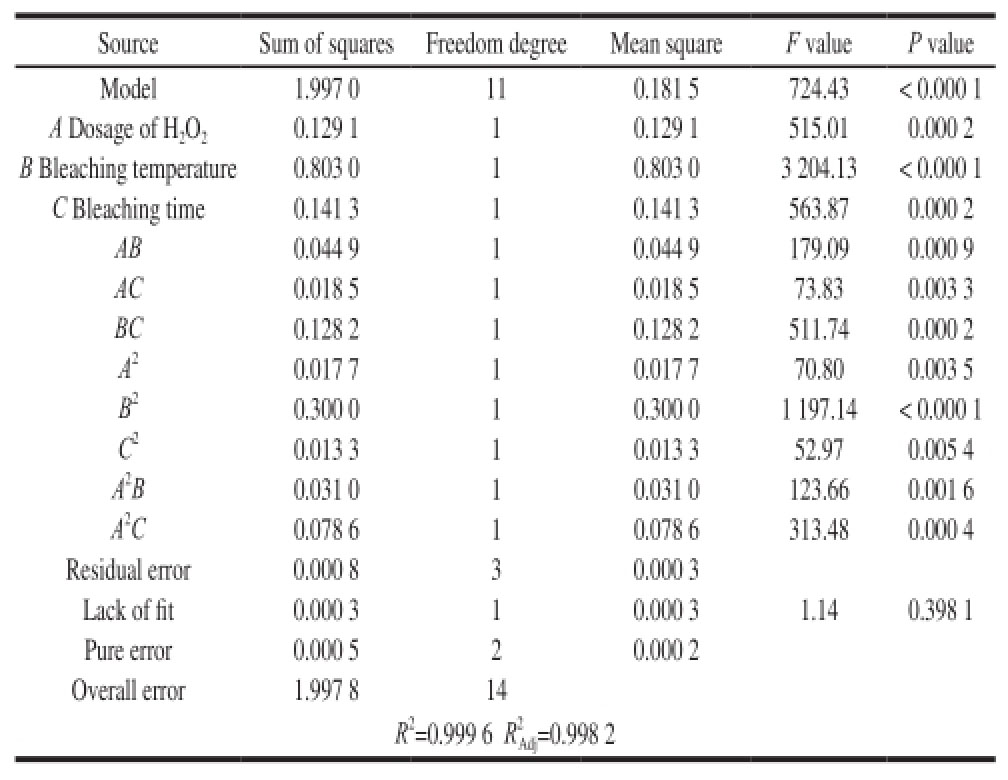
It can be observed from Table4 that the effects of all linear terms reached a significant level, i.e. P is less than 0.05. The greatest influencing factor of all linear terms on color index was bleaching temperature, followed by the dosage of H
2O
2and bleaching time, which were with equivalent influence. The influence of interaction terms on the color index followed a decreasing sequence: BC > AB > AC. For quadratic terms, the factor affecting the color index from high to low was B
2, A
2and C
2; while for the two correction terms, the influence of A
2C was greater than A
2B. All these features indicate that there were some limitations only using quadratic polynomial fitting of color index and each influence factor. It was necessary to modify the model. The response of model after modification to the variation of each influence factor was significant (model P value < 0.000 1). And P value of lack of fit was 0.398 1 > 0.05; the regression coefficient of model was R
2=0.999 6, while the Adjusted regression coefficient after modification was
![]() Both were more than 0.998. Thus, the fitting degree of model equation after modification was good.
Both were more than 0.998. Thus, the fitting degree of model equation after modification was good.
Table4 Analysis of variance of regression equation with color index as response variable
The response surfaces according to the regression equation mentioned were illustrated in Fig.7.
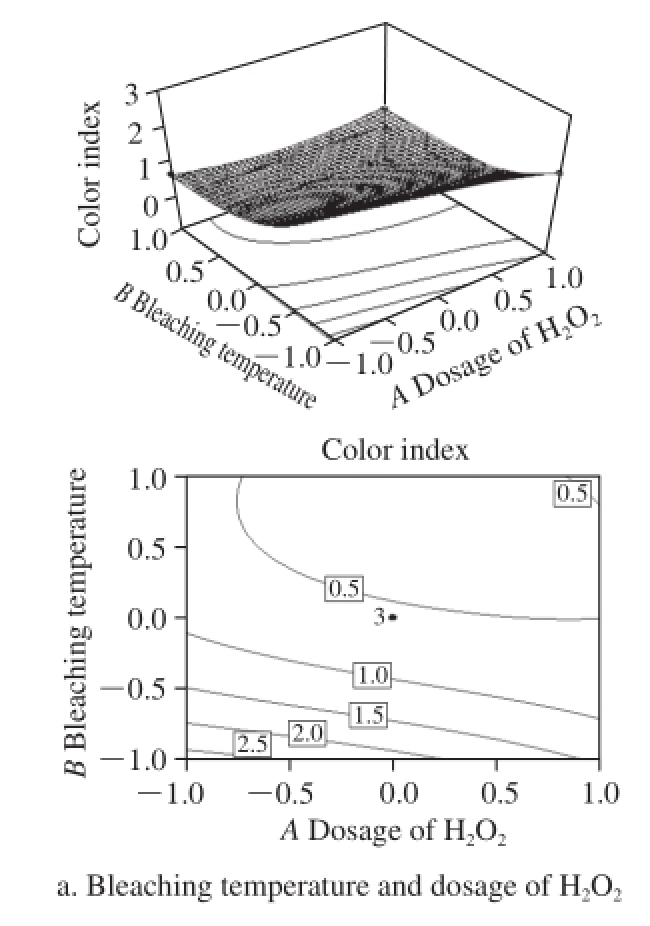
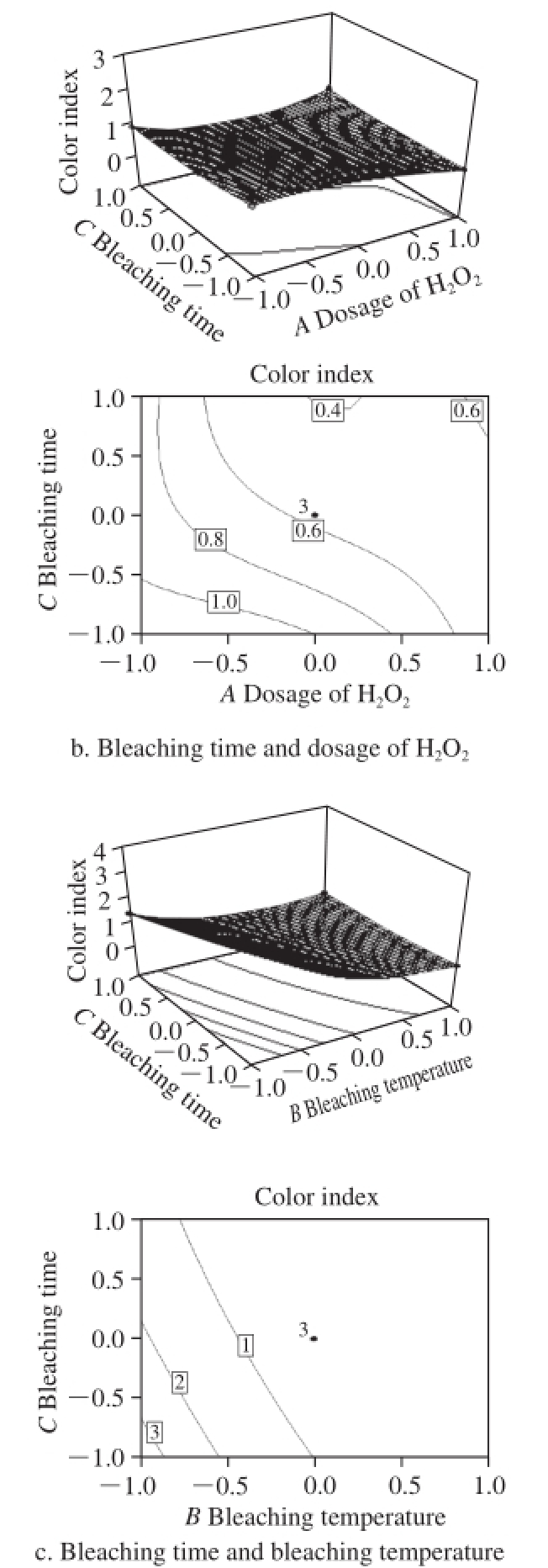
Fig.7 Response surfaces and contours showing influence of process conditions on color index
As shown in Fig.7a, there was an apparent decline in the color index of bleached shellac with increasing bleaching temperature and the dosage of H 2O 2. And the color index was less than 1 under most conditions. According to the China national standard of GB/T 8140—2009, the color index was able to meet the criteria of Grade Ⅰ refined bleached shellac. Thus, H 2O 2bleaching technique meets perfectly well the requirements of bleached shellac for color index.
Fig.7b indicates that with the increases in the dosage of H 2O 2and bleaching time, the color index was reduced gradually. It means that the damage to chromogenic materials by H 2O 2was a slow reaction process. As bleaching time and the dosage of H 2O 2increased, the bleaching cost was increased greatly, while the yield was reduces sharply because of the increasing damage of H 2O 2to shellac resin. Thereby, it is crucial for TCF bleaching technique to improve the utilization and bleaching activity of H 2O 2and to shorten bleaching time.
From Fig.7c, it can be obtained that the effect of bleaching temperature on color index increased first and followed by a decline. As mentioned in Fig.7a, it may be associated with the increases in bleaching time and temperature. Shellac is a kind of polyester matter with long carbon chain macromolecule made of polyhydroxy and carboxyl, which is more likely to have polymerization reaction with the effect of light and heat. Although the activity polymerized in Na 2CO 3alkali liquor was passivated partly, the reaction may still produce some shellac with the rising temperature and extending bleaching time so that the color was darkened.
2.3 Calculation of optimum condition
The yield and color index of bleached shellac were employed as response values in Design-Expert 8.0.6 Software for optimization. And the optimized regions of every single condition were set as follows: the dosage of H 2O 2was 2-4 mL/g; bleaching temperature was from 70 to 90 ℃, and bleaching time was 5 to 7 hours. Herein, the temperature was based on the results of optimization experiment that higher bleaching temperature was beneficial to improve the bleaching activity of H 2O 2. In addition, the optimal range was set between 0.40 and 0.80 on the premise that the color index could meet the requirement of Grade Ⅰ refined bleached shellac, namely, the color index was less than 1.0. Moreover, the optimization goal of the yield of bleached shellac was set as the maximum value. When all the requirements are met at the same time, the optimum conditions for TCF bleaching technique of shellac were as follows: the concentration of shellac in the bleaching liquor was 0.08 g/mL; the dosage of H 2O 2was 2.09 mL/g; bleaching temperature was at 80 ℃; bleaching time was 7 h and the dosage of stabilizer was 3.125% (based on the mass of shellac). And the optimal color index and the yield of bleached shellac were 0.80 and 82.96% respectively.
2.4 Verification experiment
The optimum technological conditions obtained in 2.3 were as flows: the concentration of bleaching liquor, 0.08 g/mL; the dosage of H 2O 2, 2.09 mL/g; bleaching temperature, 80 ℃; bleaching time, 7 hours, and the dosage of stabilizer, 3.125%. The color index of bleached shellac was 0.979
(less than 1.0) and the yield was 82.03%, with error of 0.80% and 1.12% less than 2%. All the value achieved the expected target. Evidently, these optimum conditions were feasible.
2.5 Contrastive analysis between H 2O 2and sodium hypochlorite bleached shellac
2.5.1 Qualitative indexes for chlorine-free bleached shellac
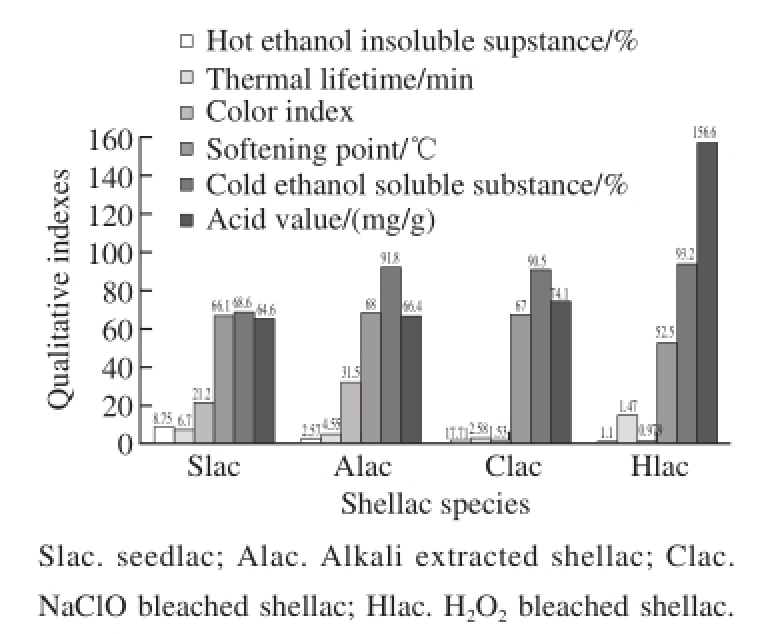
Fig.8 Comparative analysis of quality indexes of shellac bleached by four different preparative methods
Fig.8 demonstrates that comparing with sodium hypochlorite bleached shellac, some qualitative indexes of H 2O 2bleached shellac, such as hot ethanol insoluble substance, color index, softening point, etc. reduced while some were increased, for instance, cold ethanol soluble substance, thermal lifetime and acid value, etc. The improvement of alcohol-soluble and reduction of softening point may be associated with the change of intermolecular forces of shellac resin in bleaching process. And the decreasing color index shows that H 2O 2bleaching technique can totally meet the demands of China national standard. Meanwhile, the increase in acid value indicates that the quantity of carboxyl of H 2O 2bleached shellac significantly increases, which may result in the great increase of thermal lifetime mainly. In details, it is because the increase of carboxyl can significantly enhance the intramolecular and intermolecular forces in shellac and also make the forces more complex. However, only when it is heated with higher temperature and for longer time, can the coupling of intermolecular forces be broken and polymerization transition happen.
2.5.2 FTIR analysis of the two bleached shellac
Fig.9 showed that there was little difference in infrared absorption spectrums of these two bleached shellac and alkali extracted shellac. The stretching vibration absorption peaks of hydroxyl and hydroxyl C-O bond were 3 425 cm -1and 1 034 cm -1. The absorption peaks of C-H stretching vibration of methyl and methylene were 2 932 cm -1and 2 857 cm -1. The absorption peak of formation vibration of CH 3was 1 379 cm -1. The stretching vibration absorption peak of carbonyl was 1 711 cm -1. The absorption peaks of stretching vibration of C=C framework on aromatic ring of pigment were 1 635 cm -1and 1 466 cm -1. The absorption peaks of formation vibration in C-H bond surface of olefin and carboxylic acid O-H bond were 1 379 cm -1and 1 413 cm -1. The absorption peaks of carboxylic acid C-O bond and C-H formation vibration of C=C double bond were 1 254 cm -1and its splitting peak. The absorption peaks of C-H formation vibration for aromatic ring of pigment and multiple CH 2formation vibration were 780, 723 cm -1and their splitting peaks. In conclusion, the main functional groups obtained by the two bleaching techniques were similar. It means that both bleaching techniques just properly modified part of functional groups and active sites rather than changing the main structure of shellac resin.
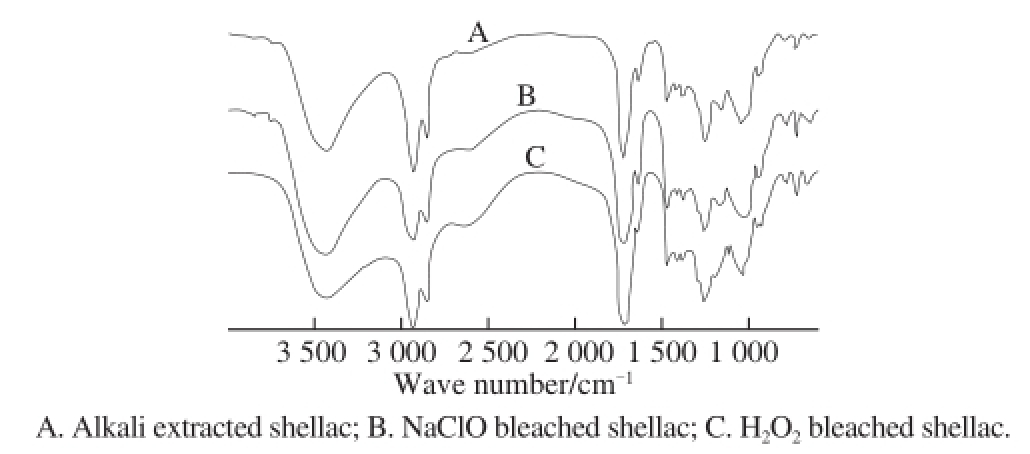
Fig.9 FTIR analysis of alkali extracted shellac, NaClO bleached shellac and H 2O 2bleached shellac
2.5.3 DSC analysis of the two bleached shellac
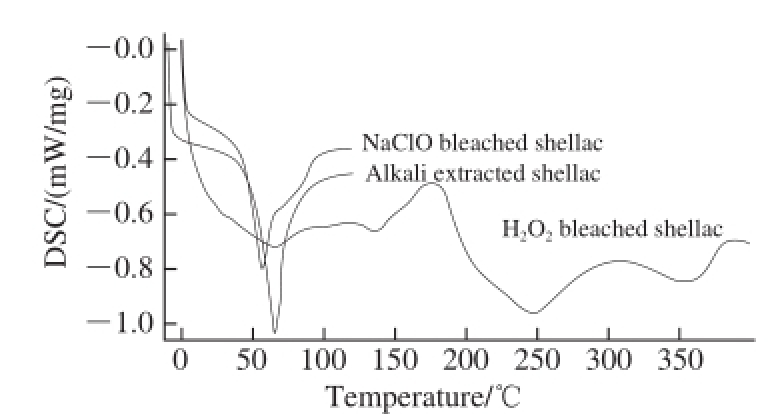
Fig.10 DSC analysis of alkali extracted shellac, NaClO bleached shellac and H 2O 2bleached shellac
Fig.10 reflected that the initial and peak points of melting of sodium hypochlorite and H 2O 2bleached shellac were lower than those of alkali extracted shellac, especially the peak points of melting. The peak points of melting of alkali extracted shellac, sodium hypochlorite and H 2O 2bleached shellac were at 66.1, 56.7 ℃ and 65.1 ℃respectively, while the initial points of melting of them were 52.7, 45.4 ℃ and 29.6 ℃ correspondingly. The variation
characteristics were consistent with those of softening point in 2.5.1. Visibly, the thermal properties of shellac were changed in the both bleaching techniques, which may be related with the change of intermolecular forces of bleached shellac induced by the degradation or oxidation of shellac by bleaching agent during bleaching. In this sense, it is significant to figure out the reaction mechanism of H 2O 2bleached shellac for further study to indentify the reasons of destruction of shellac in the bleaching process.
A new TCF bleaching technique of shellac was developed. The influences to bleaching effect of the factors, including the concentration of shellac bleaching liquor, the dosage of H 2O 2, bleaching temperature, bleaching time, the dosage of stabilizer, were investigated on the bleaching technique. Besides, the optimum conditions of TCF bleaching technique were obtained by optimizing experiment design as follows: the concentration of shellac in bleaching liquor, 0.08 g/mL; dosage of H 2O 2, 2.09 mL/g; bleaching temperature, 80 ℃, bleaching time, 7 hours and the dosage of stabilizer, 3.125% (based on the mass of shellac). The optimization results showed the color index of 0.979 and the yield of bleached shellac for 82.03%.
Through contrastive analysis of qualitative indexes, infrared spectrum and DSC curves, results indicated that there was no significant difference being found between H 2O 2and sodium hypochlorite bleached shellac in terms of in their main structures. Moreover, for qualitative indexes, the H 2O 2bleached shellac were better than sodium hypochlorite bleached shellac: its indexes such as the hot ethanol insoluble substance, cold ethanol soluble substance, color index, thermal lifetime were inproved while while some indexes including, softening point and acid value might be worse.
In light of major applications of shellac, the H 2O 2bleached shellac exhibited advantages such as avoidance of combined chlorine, improvement of alcohol-soluble property and thermal lifetime, and reduction of color index. To some extent, the increase of acid value was able to increase the hydrophilicity of shellac. However, its application is limited in some fields due to the lower softening point. Thus, the future research of TCF bleaching shellac is expected to focus on improvement of the softening point of H 2O 2bleached shellac.
References:
[1] SCHELL D, BEERMANN C. Fluidized bed microencapsulation of Lactobacillus reuteri with sweet whey and shellac for improved acid resistance and in vitro gastro-intestinal survival[J]. Food Research International, 2014, 62: 308-314. DOI:10.1016/j.foodres.2014.03.016.
[2] AL-GOUSOUSA J, PENNINGB M, LANGGUTHA P. Molecular insights into shellac film coats from different aqueous shellac salt solutions and effect on disintegration of enteric-coated soft gelatin capsules[J]. International Journal of Pharmaceutics, 2015, 484(1/2): 283-291. DOI:10.1016/j.ijpharm.2014.12.060.
[3] WANG J Y. The exploitation and use of butea gum in aneient china[J]. China Historical Materials of Science and Technology, 2000, 21(3): 222-227.
[4] ZHANG H, YU L S, ZHENG H, et al. Study on the kinetics and optimization of technological process of microwave-vacuum drying of bleached shellac[J]. Advanced Materials Research, 2011, 418/419/420: 94-101. DOI:10.4028/www.scientific.net/AMR.
[5] LI K, ZHANG H, ZHENG H, et al. Process optimization for ultrasonic-enhanced washing of modified lac[J]. Food Science, 2011, 32(18): 102-107.
[6] PATEL A R, RAJARETHINEM P S, GREDOWSKA A, et al. Edible applications of shellac oleogels: spreads, chocolate paste and cakes[J]. Food and Function, 2014, 5: 645-652. DOI:10.1039/C4FO00034J.
[7] LIAO Y L, PENG J H, LIU Z H. National and international seedlac processing development and its trend[J]. Science Silvae Sinicae, 2007, 43(7): 93-99. DOI:10.3321/j.issn:1001-7488.2007.07.016.
[8] CHEN X M, CHEN Y Q, ZHANG H, et al. Lac insect cultivation and lac processing[M]. Beijing: China Forestry Press, 2008: 44-46.
[9] JO W S, SONG H Y, SONG N B, et al. Quality and microbial safety of ‘Fuji’ apples coated with carnauba-shellac wax containing lemongrass oil[J]. LWT-Food Science and Technology, 2014, 55(2): 490-497. DOI:10.1016/j.lwt.2013.10.034.
[10] ALZAHRANI H, BEDIR Y, AL-HAYANI A. Efficacy of shellac, a natural product, for the prevention of wet gangrene[J]. Journal of International Medical Research, 2013, 41(3): 795-803. DOI:10.1177/0300060513483391.
[11] PATEL A R, SCHATTERMAN D, de VOS W H, et al. Preparation and rheological characterization of shellac oleogels and oleogel-based emulsions[J]. Journal of Colloid and Interface Science, 2013, 411: 114-121. DOI:10.1016/j.jcis.2013.08.039.
[12] MA L Y, GAN J, YIN N, et al. Application of nano-SiO xin fruit preservation agent[J]. Journal of Beijing Forestry University, 2004, 26(6): 98-101.
[13] GAN J, ZHANG H, MA L Y, et al. Effect of bleached shellac on quality of apple in room temperature storage[J]. Food Science, 2009, 30(24): 435-438. DOI:10.3321/j.issn:1002-6630.2009.24.102.
[14] LIMMATVAPORAT S, PANCHAPORNPON D, LIMMATVAPORAT C, et al. Formation of shellac succinate having improved enteric film properties through dry media reaction[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2008, 70(1): 335-344. DOI:10.1016/j.ejpb.2008.03.002.
[15] LIMMATVAPORAT S, LIMMATVAPORAT C, PUTTIPIPATKHACHORN S, et al. Modulation of drug release kinetics of shellac-based matrix tables by in-situ polymerization through annealing process[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2008, 69(3): 1004-1013. DOI:10.1016/ j.ejpb.2008.01.027.
[16] FARAG Y, LEOPOLD C S. Development of shellac-coated sustained release pellet formulations[J]. European Journal of Pharmaceutical Sciences, 2011, 42(4): 400-405. DOI:10.1016/j.ejps.2011.01.006.
[17] LIMMATVAPORAT S, LIMMATVAPORAT C, PUTTIPIPATKHACHORN S, et al. Enhanced enteric properties and stability of shellac films through composite salts formation[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2007, 67(3): 690-698. DOI:10.1016/j.ejpb.2007.04.008.
[18] FELIX S, MARIANNE S, TOR W, et al. Shellac as an acidic polymer for enteric coating[J]. Pharmaceutical Technology, 1999, 23(3): 146-154.
[19] TANG L Y, ZHENG H, ZHANG H. Effect of different shellac production and produce technology on characterizes of edible shellac film[J]. Science and Technology of Food Industry, 2009, 30(5): 289-292.
[20] ANTIC B, BLAGOJEVIC B, BUNCIC S. Treatment of cattle hides with shellac solution to reduce hide-to beef microbial transfer[J]. Meat Science, 2011, 88(3): 498-502. DOI:10.1016/j.meatsci.2011.01.034.
[21] CHAUHAN O P, RAJU P S, SINGH A, et al. Shellac and aloe-gelbased surface coatings for maintaining keeping quality of apple slices[J]. Food Chemistry, 2011, 126(3): 961-966. DOI:10.1016/ j.foodchem.2010.11.095.
[22] LIAO Y L, CHAI X J. Preparation of bleached low-chlorine shellac by catalytic hydrogenation and its structural characterization[J]. Chemistry and Industry of Forest Products, 2008, 28(5): 100-104. DOI:10.3321/j.issn:0253-2417.2008.05.020.
[23] ZHOU H, GAN Q G. Study on bleaching mechanism of chain component in lac resin-aleuritic acid[J]. Chemistry and Industry of Forest Products, 1994, 14(1): 9-13.
[24] HA C Y, WANG D X. Storage properties of bleached lac IV. Kinetics of halogenation of cyclic parts in lac resin[J]. Chemistry and Industry of Forest Products, 1993, 13(2): 103-108.
[25] WANG Y M, CHEN J H, WU D M, et al. Test methods of lac products: GB/T 8143—2008[S]. Beijing: General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China(AQSIQ), 2008.
[26] SHI L, ZHAO H, CHEN X M, et al. Bleached shellac: GB/T 8140—2009[S]. Beijing: General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China(AQSIQ), 2009.
响应面试验优化紫胶全无氯漂白工艺及其产物性能
李 坤 1,2,郑 华 1,2,张雯雯 1,李 凯 1,徐 涓 1,张 弘 1,2,*
(1.中国林业科学研究院资源昆虫研究所,国家林业局特色森林资源工程技术研究中心,云南 昆明 650224;2.国家林业局资源昆虫培育与利用重点实验室,云南 昆明 650224)
摘 要:为避免传统含氯漂白紫胶的潜在风险,提高漂白紫胶在食品、医药等领域的使用安全性,本研究开发了紫胶的全无氯漂白工艺,采用响应面法研究了紫胶漂液质量浓度、H 2O 2用量、漂白温度、漂白时间、稳定剂用量等因素对漂白效果的影响,得出了紫胶全无氯漂白的最佳工艺条件:漂液中紫胶质量浓度0.08 g/mL、H 2O 2用量2.09 mL/g(以紫胶质量计)、漂白温度80 ℃、漂白时间7 h、稳定剂用量3.125%(以紫胶质量计)。在此基础上,通过傅里叶红外光谱、差示扫描量热、质量指标测定等方法对所得H 2O 2漂白胶进行了表征分析,结果发现:与次氯酸钠漂白胶和碱提紫胶相比,H 2O 2漂白胶分子整体结构基本一致,H 2O 2漂白胶热寿命、冷乙醇可溶物、酸值等指标明显提高,热乙醇不溶物、软化点等指标明显下降,颜色指数可达0.979(<1.0),漂白胶得率为82.03%。所得无氯漂白紫胶可作为优良的食品添加剂和加工原材料。
关键词:紫胶;全无氯漂白;工艺;模型与优化
中图分类号:TQ351.7;TS202.3
文献标志码:A
文章编号:1002-6630(2016)22-0041-11
引文格式:
收稿日期:2016-04-22
基金项目:国家高技术研究发展计划(863计划)项目(2014AA021801);
作者简介:李坤(1987—),男,助理研究员,硕士,主要从事天然资源化学与利用研究。E-mail:likunkm@163.com
DOI:10.7506/spkx1002-6630-201622007
中央级公益性科研院所基本科研业务费专项(riricaf2014005M)
*通信作者:张弘(1963—),男,研究员,博士,主要从事林业生物资源化学与利用研究。E-mail:kmzhhong@163.com