
图1 不同组别的雌性成年子代大鼠在不同音频的超声波播放下的最长寂静时间
Fig.1 Maximal silence of adult female rat offsprings from different groups under different ultrasonic vocalizations
左满花 1,2,黄德斌 1,胡秀英 2,唐 俊 3,*
(1.湖北民族学院医学院,湖北 恩施 445000;2.四川大学华西临床医学院,四川 成都 610041;3.恩施土家族苗族自治州中心医院肾内科,湖北 恩施 445000)
摘 要:目的:探讨硫酸锌(ZnSO 4)注射液对孕期暴露于脂多糖(lipopolysaccharides,LPS)大鼠雌性成年子代的行为、神经免疫功能的影响及其机制。方法:随机将15 只孕期(孕期0~9.5 d)Wistar大鼠分为3 组,分别注射LP S和ZnSO 4(LPS+Zn组)、LPS和无菌生理盐水(saline,SAL)(LPS+SAL组)、SAL和SAL(SAL+SAL组)。产后81~86 d,从每窝选取2~3 只雌性成年子代(10~12 只/组),动情间期将大鼠放置在抑制性应激反应管道2 h,在最后5 min,播放不同音频的超声波,记录大鼠保持寂静的时间(寂静期)。超声波测试后,立即将雌性成年 子代放置在开放 场域中,测定其运动和焦虑状态。开放场域行为测试后,立即取大鼠躯干 血,采用酶联免疫吸附实验(enzyme-linked immunosorbent assay,ELIS A)测定血清皮质酮、脑源性神经营养因子(brain-derived neurotrophic factor,BDNF)水平;采用高效液相色谱法(high performance liquid chromatography,HPLC)测定大鼠下丘脑和纹状体的单胺及其代谢产物水平。结果:与LPS+SAL组、SAL+SAL组相比,LPS+Zn组的雌性成年子代大鼠呈现最大音频寂静持续时间延长(P<0.05或P<0.01)、行走距离延长(P<0.05或P<0.001)、平均速率加快(P<0.05或P<0.001)、自我梳理时间缩短(P<0.001或P<0.01)、纹状体去甲肾上腺素代谢率降低(P<0.05或P<0.01)的特征。与LPS+SAL组相比,LPS+Zn组大鼠的血清皮质酮水平降低(P<0.05)。结论:ZnSO 4注射液作用于孕期遭受感染或炎症的母体,在急性抑制应激期后,其雌性成年子代应激反应减轻。即孕期为感染的母体注射ZnSO 4也许是一个潜在的、有益的保护其子代行为的防治措施。
关键词:锌;孕期;大鼠;脂多糖;子代;应激反应
孕期9.5 d正好是Wistar大鼠子代大脑和组织器官形成的关键时期。感染与机 体的免疫系统有关,与妊娠晚期相比,妊娠早、中期的感染对子代神经系统发育有强烈影响。在妊娠早、中期,感染导致母体免疫系统激活,阻碍细胞增殖、分化、迁移、靶区选择、神经元突触成熟,甚至导致其成年子代的多形脑和行为异常 [1-4]。脂多糖(lipopolysaccharide,LPS)为含糖和脂质的化合物,是革兰氏阴性细菌细胞壁中的一种成分,也是细菌内毒素的主要成分。孕期进食太多高脂肪与高糖的食物会导致体内LPS增多,对机体产生毒性。孕期暴露于LPS的母鼠,其子代纹状体和嗅球中多巴胺能神经元的功能会被削弱 [5]。也有文献研究证实 [6-8]孕期暴露于LPS的大鼠,其子代在无外界刺激源的情况下,雌性子代的行为及血清皮质酮不会受到影响,仅雄性子代表现异常。然而以上这些异常改变仅限于孕鼠感染LPS后,其子代在自然状态下(无压力源刺激)进行的机体、神经免疫系统的监测;而对施加外界压力源刺激情况下,其雌性成年子代的行为、神经免疫系统是否发生改变目前没有研究报道。
锌(Zn)是生物所必需的一种微量元素,对生物的生长发育、免疫功能等起着重要的作用 [9-10]。ZnSO 4属于无机锌,较有机锌难以被吸收 [11],故不易导致机体中毒。因此,本实验探究以ZnSO 4注射液作用孕期暴露于LPS的大鼠,将其成年雌性子代在动情间期放置于急性抑制应激管道后,播放不同音频的超声波,观察ZnSO 4注射液对其雌性成年子代应激反应的影响,探讨其行为、神经调节机制。
1.1 动物、材料与试剂
15 只孕期Wistar大鼠,由湖北省实验动物研究中心提供(生产许可证号:SCXK(鄂)2008-0005 ;使用许可证号:SYXK(鄂)2008-0014),体质量226~266 g。
ZnSO 4、LPS(10 mg,2~8 ℃冷藏保存) 美国Sigma公司。
无菌生理盐水(saline,SAL,0.9%) 上海百特医疗用品有限公司;脑源性神经营养因子(brain-derived neurotrophic factor,BD NF)酶联免疫吸附(enzymelinked immunosorbent assay,ELISA)试剂盒 南京建成生物工程研究所。
1.2 仪器与设备
抑制性应激反应器材:直径5 cm、长度可改变、两端封闭,尾部有无数个小孔的可塑形圆柱型管道;开放场域:直径90 cm、高28 cm,四周涂满可水洗灰色颜料、圆形无盖的木制箱子。
HP1050型高效液相色谱(high performance liquid chromatography,HPLC)仪 美国Agilent公司;超声波播放器 美国诺达思信息科技有限公司;FDR-AXP35摄影机 日本索尼公司。
1.3 动物及分组
将15 只孕期Wistar大鼠随机分为3 组,每组5 只:两实验组孕鼠在孕期0~9.5 d(即从受孕到孕期9.5 d)均先接受LPS(100 μg/(kg•d))腹腔注射 [12],其中一组1 h后接受ZnSO 4(2 mg/(kg•d))颈后部皮下注射 [13-14](LPS+Zn组),另一组1 h后接受SAL(0.2 mL/(100 g•d))颈后部皮下注射(LPS+SAL组),均连续进行9 d;对照组孕鼠同样在孕期0~9.5 d先接受SAL(0.2 mL/(100 g•d))腹腔注射,1 h后再次接受SAL(0.2 mL/(100 g•d))颈后部皮下注射(SAL+ SAL组),连续进行9 d。孕鼠产后81~86 d,从每窝选取2~3 只成年雌性子代用来评估其应激反应行为 [15]。
1.4 方法
1.4.1 雌性成年子代应激行为超声波检测
通过阴道涂片检测,确定雌性成年子代动情期,在动情后期或动情间期,逐次把每只雌性子代放到抑制性应激反应管道2 h [16-17](直径5 cm,长度可变)。在抑制期最后5 min,开始用超声波播放器(22 kHz)播放诸如掠肉或足底电击等令雌性成年子代厌恶的音乐 [18-19],通过超声波软件(距离抑制性应激管道1 cm)自动记录最低频率(kHz)和最大音频寂静持续时间(s)并进行分析。
1.4.2 雌性成年子代场域行为检测
超声波检测后,立即进行场域实验:将3 组中每只雌性成年子代单独、逐次放置于木制圆形无盖的箱子里(直径90 cm、高28 cm),木制圆形的箱子放置在一个光线昏暗的小房间内,距离房间地面100 cm任一高处放置一架摄影机,借助Ethovision软件自动或人工记录5 min期间内雌性成年子代的各种运动和焦虑参数 [20-21]:行走距离(cm)、平均速率(cm/s)、自我梳理时间(s)、喂养次数。
1.4.3 雌性成年子代血清皮质酮、BDNF、单胺类及其代谢产物含量检测
开放场域行为检测后,立即取雌性成年子代躯干血液,置离心管内,室温条件下4 000 r/min离心10 min,取血清-70 ℃保存。一式两份,按照ELISA试剂盒说明书方法测定皮质酮和BDNF含量。
采用HPLC法分析雌性成年子代下丘脑和纹状体中的单胺类及其代谢产物含量。所测代谢产物有:去甲肾上腺素(norepinephrine,Nor)和香草扁桃酸(vanilmandelic acid,VMA)、3-甲氧基-4-羟基苯基乙二醇(3-methoxy-4-hydroxy-phenylglycol,MOPEG)、多巴胺(dopamine,DA)和3,4-二羟基苯乙酸(3,4-dihydroxyphenylacelic acid,DOPAC)、高香草酸(homovanillic acid,HVA)、5-羟色胺(5-hydroxytryptamine,5-HT)和5-羟吲哚乙酸(5-hydroxy indole acetic acid,5-HIAA)。
1.5 统计分析方法
所有数据均以±s表示,采用SPSS 17.0统计软件对数据进行统计学分析,以单因素方差分析和组间SNK(Student-Newman-Keuls)法检验两两之间差异性,P<0.05为差异显著,P<0.01为差异极显著,P<0.001为差异高度显著。
2.1 超声波检测结果

图1 不同组别的雌性成年子代大鼠在不同音频的超声波播放下的最长寂静时间
Fig.1 Maximal silence of adult female rat offsprings from different groups under different ultrasonic vocalizations
如图1所示,与LPS+SAL组相比,LPS+Zn组雌性成年子代的最大音频寂静持续时间显著延长(P<0.05);与SAL+SAL组相比,LPS+Zn组雌性成年子代的最大音频寂静持续时间极显著延长(P<0.01)。说明在产前以ZnSO 4注射液作用于孕期暴露于LPS的母鼠,其雌性成年子代在急性抑制应激反应后,在相同音频超声波的刺激下最大音频寂静持续时间延长,降低了其子代的应激反应行为。
2.2 场域行为检测结果

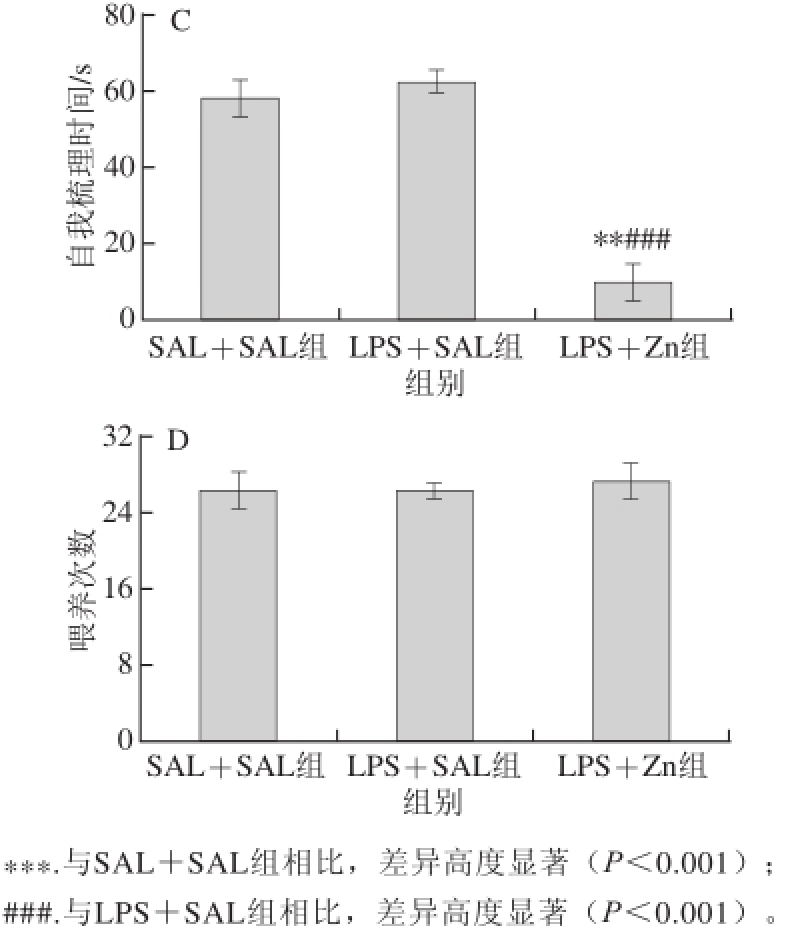
图2 不同组别的成年雌性子代大鼠在开放场域中行为检测结果
Fig.2 Open field behavior of adult female rat offspring from different groups
如图2所示,与LPS+SAL组、SAL+SAL组相比,LPS+Zn组雌性成年子代行走距离和平均速率明显增大(P<0.05或P<0.001),自我梳理时间明显缩短(P<0.001或P<0.01),说明以ZnSO 4注射液作用孕期暴露于LPS的母鼠,可以增加其雌性成年子代行走距离、提高其行走的平均速率,缩短其冰冻行为的时间 [22]。
2.3 雌性成年子代血清皮质酮和BDNF含量

图3 不同组别成年雌性子代大鼠的血清皮质酮和总BDNFF含量
Fig.3 Serum corticosterone and total BDNF levels in adult female rat offspring from different groups
如图3所示,与LPS+SAL组相比,LPS+Zn组雌性成年子代血清皮质酮含量显著减少(P<0.05),说明在外界刺激源改变的情况下,ZnSO 4注射液抑制了孕鼠下丘脑-垂体-肾上腺(hypothalamic-pituitary-adrenal,HPA)轴活性,降低雌性成年子代血清皮质酮的分泌。
2.4 雌性成年子代下丘脑和纹状体中的单胺类及其代谢产物含量
表1 雌性成年子代大鼠不同组织中单胺类及其代谢产物水平的变化(x±s =8)
Table 1 The levels of monoamine and its metabolites in different tissues (x ± s, = 8)

?
注:表中数据以组织湿质量计;*. 与SAL+SAL组相比,差异显著(P<0.05);**.与SAL+SAL组相比,差异极显著(P<0.01);#. 与LPS+SAL组相比,差异显著(P<0.05)。
由表1可知,与SAL+SAL组相比,LPS+Zn组雌性成年子代纹状体VMA含量和VMA/Nor明显下降(P<0.01或P<0.05);与LPS+SAL组相比,LPS+Zn组雌性成年子代V M A代谢水平下降,差异显著(P<0.05)。说明以ZnSO 4注射液作用孕期暴露于LPS的母鼠,可以降低其雌性子代纹状 体中Nor代谢产物VMA的水平和VMA/Nor。
孕期母体暴露于LPS,产生的促炎性细胞因子会在母体的血液循环和胎盘之间流动 [23-25]。相关的文献研究证实 [26-27]:孕期暴露于LPS会导致大鼠病态行为、胎盘组织受损、受精卵种植(着床)损失率高、幼崽出生率降低、子代的行为和神经免疫系统长期或短期受到一定的影响。Coyle的团队研究报道 [28-29]:孕期小鼠母体暴露于LPS,致使其体内血清锌含量较低,易导致其子代畸形;给母体补充适量的锌可防止子代不良基因遗传和行为异常。
锌属于哺乳动物体内最重要微量元素之一,参与一系列的生理、生化过程,如细胞的增殖和分化、生长和发育、参与酶的活性调解等 [30]。孕期母体缺乏锌会导致子代一些行为、功能异常的表现,如学习和记忆能力的缺失 [31]、成年期心血管和肾脏系统疾病的发生 [32-33]。孕期在饮食中补充锌或进行ZnSO 4的腹腔注射,有益于其子代健康发育,包括认知发展 [34]、合适体质量保持 [35]、胎儿心脏结构和功能的成熟 [36]。对其子代施加外界刺激源的情况下,加上产前母体暴露于过多的LPS,引起母体神经免疫系统激活,释放细胞因子 [37-38],激活HPA轴,导致子代脑中糖皮质激素产生增多,与本研究中LPS+SAL组雌性成年子代血清皮质酮水平最高相符;加上机体LPS促进金属硫蛋白(metallothionein,MT)产生,引起母体及胎儿体内血清锌含量降低,导致其体内自由基产生过多,引起机体过氧化应激反应,子代在其宏观上表现为场域行为中自我梳理时间延长、明显的焦虑状态,与本研究中LPS+SAL组雌性成年子代表现相符。锌抗氧化和抗凋亡的特性可限制或抑制LPS作用于机体所导致的不良反应 [39-40]。本研究显示:LPS+Zn组雌性成年子代的音频较低、寂静持续时间最长、行走距离最长、平均速率最高、自我梳理时间最短,表示在外界刺激源的情况下,其子代应对应激反应行为最强。这与相关研究报道相符 [41]:ZnSO 4注射液治疗孕期暴露于LPS的大鼠,通过血脑屏障和胎盘,调节其子代血清锌含量,锌诱导MT产生ZnMT复合物,在外界刺激源存在的情况下,拮抗机体遭受甲基汞产生的自由基对细胞膜通透性的破坏,降低膜外乳酸脱氢酶的活性,抑制自由基对机体造成的过氧化损伤,节省机体能量,减轻机体的过氧化应激行为。
BDNF是存在于大脑、中枢神经系统及外周血液中的一种小分子质量蛋白质,它管理着神经元的存活、成型、发育和功能,在突触产生和可塑性方面也起着关键作用 [42]。腹腔注射ZnSO 4 [43]或饮食补充含锌的食物 [44]均可以影响BDNF的表达。相关研究显示:锌缺乏会损伤星形胶质细胞,且星形胶质细胞又是许多BDNF分泌的来源 [45];锌缺乏还会导致子代体内BDNF分泌量降低 [46],与本研究中LPS+SAL组雌性成年子代脑源性BDNF水平最低的结果相符。3 组雌性成年子代血液BDNF含量比较无统计学意义,因此目前还不能明确产前注射ZnSO 4是否利于成年子代在施加外界应激源挑战的情况下BDNF的表达。
锌在脑中的分布是不均一的,以边缘系统、皮质部、齿状回和海马中含量较高,尤以海马结构和大脑皮层含量最高。下丘脑是调节内脏活动和内分泌活动的较高级神经中枢所在。纹状体除接受来自同侧新纹状体的神经纤维以外,还接受来自大脑皮层的神经纤维,其与随意运动的稳定、肌张力的维持以及肢体姿势的调节活动密切相关。测量神经系统激素代谢 产物的水平和神经递质的比例,被认为是检测此系统活性和激素转换率的一种有效手段 [47]。越在动物发育的 早期,神经系统的可塑性也越明显。锌作为体内200多种酶和功能蛋白质的组成成分,势必会影响神经细胞的结构和功能,在宏观上表现为行为学的变化,即越在动物发育的早期,影响也越大。故本实验在大鼠孕期0~9.5 d对其腹腔注射ZnSO 4溶液,研究其雌性成年子代行为的表现。本实验借助HPLC法检测大鼠脑组织单胺类及其代谢产物含量,结果显示,产前以ZnSO 4注射液作用于LPS+Zn组大鼠,其雌性成年子代纹状体中VMA水平和VMA/Nor下降。这一结果很好地支持了场域行为和血清HPA轴活性检测结果:ZnSO 4溶液通过胎盘提高了LPS +Zn组大鼠雌性成年子代机体血液中锌的含量,间接抑制了LPS对HPA的激活,导致神经免疫系统激素分泌量下降,降低了雌性成年子代的应激反应。
本实验研究结果显示,产前以ZnSO 4注射液作用孕期暴露于LPS的大鼠,其成年雌性子代在受到急性抑制应激后,其应激反应降低,从而减轻了对机体的不利影响。因此,母体若在孕期遭受感染或炎症侵袭,及早补充微量元素锌可能是一个潜在的有意 义措施。本实验的结论为孕期妇女发生感染后防止胎儿畸形的研究提供了一定参考,即及早口服硫酸锌也许是一个较好的举措。
参考文献:
[1] MEYER U, NYFFELER M, ENGLER A, et al. The time of prenatal immune challenge determines the specifi city of infl ammation-mediated brain and behavioral pathology[J]. Journal of Neuroscience, 2006, 26(18): 4752-4762. DOI:10.1523/JNEUROSCI.0099-06.2006.
[2] MEYER U, YEE B K, FELDON J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse?[J]. Neuroscientist, 2007, 13(3): 241-256. DOI:10.1177/1073858406296401.
[3] SAMUELSSON A M, JENNISCHE E, HANSSON H A, et al. Prenatal exposure to interleukin-6 results in infl ammatory neurodegeneration in hippocampus with NMDA/GABAA dysregulation and impaired spatial learning[J]. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2006, 290(5): R1345-R1356. DOI:10.1152/ajpregu.00268.2005.
[4] SHI L, FATEMI S H, SIDWELL R W, et al. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring[J]. Journal of Neuroscience, 2003, 23(1): 297-302.
[5] KIRSTEN T B, LIPPI L L, BEVILACQUA E, et al. LPS exposure increases maternal corticosterone levels, causes placental injury andincreases IL-1β levels in adult rat offspring: relevance to autism[ J]. PLoS ONE, 2013, 8(12): e82244. DOI:10.1371/journal.pone.0082244.
[6] KIRSTEN T B. Lipopolissacarídeo no início do período pré-natal como modelo experimental de autismo e prejuízos dopaminérgicos estriatais[D]. São Paulo: Universidade de São Paulo, 2012.
[7] KIRSTEN T B, CHAVES-KIRSTEN G P, CHAIBLE L M, et al. Hypoactivity of the central dopaminergic system and autisticlike behavior induced by a single early prenatal exposure to lipopolysaccharide[J]. Journal of Neuroscience Research, 2012, 90(10): 1903-1912. DOI:10.1002/jnr.23089. Epub 2012 Jun 20.
[8] TAYLOR P V, VEENEMA A H, PAUL M J, et al. Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats[J]. Biology of Sex Differences, 2012, 3(1): 1-9. DOI:10.1186/2042-6410-3-15.
[9] 龚毅, 胡晓波, 彭丽霞, 等. 锌氨基酸螯合物的抑菌活性研究[J]. 食品科学, 2009, 30(17): 84-87.
[10] 李川, 朱科学, 聂少平, 等. 苏氨酸锌对糖尿病大鼠肝脏损伤的保护作用[J]. 食品科学, 2011, 32(19): 198-200.
[11] 于昱. 不同形态锌在肉仔鸡小肠中的吸收特点及机理研究[D]. 北京: 中国农业科学院, 2008.
[12] LENCZOWSKI M J P, van DAM A M, POOLE S, et al. Role of circulating endotoxin and interleukin-6 in the ACTH and corticosterone response to intraperitoneal LPS[J]. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 1997, 273(6): R1870-R1877.
[13] CHUA J S C, COWLEY C J, MANAVIS J, et al. Prenatal exposure to lipopolysaccharide results in neurodevelopmental damage that is ameliorated by zinc in mice[J]. Brain, Behavior, and Immunity, 2012, 26(2): 326-336.
[14] SUMMERS B L, ROFE A M, COYLE P. Prenatal zinc treatment at the time of acute ethanol exposure limits spatial memory impairments in mouse offspring[J]. Pediatric Research, 2006, 59(1): 66-71. DOI:10.1203/01.pdr.0000190573.23893.13.
[15] GIOVANOLI S, ENGLER H, ENGLER A, et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice[J]. Science, 2013, 339: 1095-1099. DOI:10.1126/ science.1228261.
[16] BUYNITSKY T, MOSTOFSKY D I. Restraint stress in biobehavioral research: recent developments[J]. Neuroscience & Biobehavioral Reviews, 2009, 33(7): 1089-1098. DOI:10.1016/ j.neubiorev.2009.05.004.
[17] GUSTAFSSON J K, GREENWOOD-VAN MEERVELD B. Amygdala activation by corticosterone alters visceral and somatic pain in cycling female rats[J]. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2011, 300(6): G1080-G1085. DOI:10.1152/ajpgi.00349.2010.
[18] RHODES M E, KENNELL J S, BELZ E E, et al. Rat estrous cycle influences the sexual diergism of HPA axis stimulation by nicotine[J]. Brain Research Bulletin, 2004, 64(3): 205-213. DOI:10.1016/ j.brainresbull.2004.06.011.
[19] ANTONIADIS E A, MCDONALD R J. Discriminative fear conditioning to context expressed by multiple measures of fear in the rat[J]. Behavioural Brain Research, 1999, 101(1): 1-13. DOI:10.1016/ S0166-4328(98)00056-4.
[20] TAKAHASHI N, KASHINO M, HIRONAKA N. Structure of rat ultrasonic vocalizations and its relevance to behavior[J]. PLoS ONE, 2010, 5(11): e14115. DOI:10.1371/journal.pone.0014115.
[21] PATTI C L, FRUSSA-FILHO R, SILVA R H, et al. Behavioral characterization of morphine effects on motor activity in mice[J]. Pharmacology Biochemistry and Behavior, 2005, 81(4): 923-927. DOI:10.1016/j.pbb.2005.07.004.
[22] SUDAKOV S K, NAZAROVA G A, ALEKSEEVA E V, et al. Estimation of the level of anxiety in rats: differences in results of openfi eld test, elevated plus-maze test, and Vogel’s confl ict test[J]. Bulletin of Experimental Biology and Medicine, 2013, 155(3): 295-297. DOI:10.1007/s10517-013-2136-y.
[23] NOSEK K, DENNIS K, ANDRUS B M, et al. Context and straindependent behavioral response to stress[J]. Behavioral and Brain Functions, 2008, 4(23): 1-8. DOI:10.1186/1744-9081-4-23.
[24] ASHDOWN H, DUMONT Y, NG M, et al. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia[J]. Molecular Psychiatry, 2006, 11(1): 47-55. DOI:10.1038/sj.mp.4001748.
[25] CAI Z, PAN Z L, PANG Y I, et al. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration[J]. Pediatric Research, 2000, 47(1): 64-72. DOI:10.1203/00006450-200001000-00013.
[26] URAKUBO A, JARSKOG L F, LIEBERMAN J A, et al. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain[J]. Schizophrenia Research, 2001, 47(1): 27-36. DOI:10.1016/S0920-9964(00)00032-3.
[27] KIRSTEN T B, TARICANO M, MAIORKA P C, et al. Prenatal lipopolysaccharide reduces social behavior in male offspring[J]. Neuroimmunomodulation, 2010, 17(4): 240-251. DOI:10.1159/000290040.
[28] CAREY L C, BERBÉE P L, COYLE P, et al. Zinc treatment prevents lipopolysaccharide-induced teratogenicity in mice[J]. Birth Defects Research Part A: Clinical and Molecular Teratology, 2003, 67(4): 240-245. DOI:10.1002/bdra.10035.
[29] COYLE P, TRAN N, FUNG J N T, et al. Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy[J]. Behavioural Brain Research, 2009, 197(1): 210-218. DOI:10.1016/ j.bbr.2008.08.022.
[30] MARET W, SANDSTEAD H H. Zinc requirements and the risks and benefits of zinc supplementation[J]. Journal of Trace Elements in Medicine and Biology, 2006, 20(1): 3-18. DOI:10.1016/ j.jtemb.2006.01.006.
[31] YU X D, JIN L M, ZHANG X H, et al. Effects of maternal mild zinc defi ciency and zinc supplementation in offspring on spatial memory and hippocampal neuronal ultrastructural changes[J]. Nutrition, 2013, 29(2): 457-461. DOI:10.1016/j.nut.2012.09.002.
[32] TOMAT A L, de los ÁNGELES COSTA M, ARRANZ C T. Zinc restriction during different periods of life: influence in renal and cardiovascular diseases[J]. Nutrition, 2011, 27(4): 392-398. DOI:10.1016/j.nut.2010.09.010.
[33] TOMAT A L, VEIRAS L C, AGUIRRE S, et al. Mild zinc defi ciency in male and female rats: early postnatal alterations in renal nitric oxide system and morphology[J]. Nutrition, 2013, 29(3): 568-573. DOI:10.1016/j.nut.2012.09.008.
[34] PIECHAL A, BLECHARZ-KLIN K, PYRZANOWSKA J, et al. Maternal zinc supplementation improves spatial memory in rat pups[J]. Biological Trace Element Research, 2012, 147(1/3): 299-308. DOI:10.1007/s12011-012-9323-y.
[35] SAAKA M, OOSTHUIZEN J, BEATTY S. Effect of prenatal zinc supplementation on birthweight[J]. Journal of Health, Population, and Nutrition, 2009, 27(5): 619-631. DOI:10.3329/jhpn.v27i5.3638.
[36] MERIALDI M, CAULFIELD L E, ZAVALETA N, et al. Randomized controlled trial of prenatal zinc supplementation and the development of fetal heart rate[J]. American Journal of Obstetrics and Gynecology, 2004, 190(4): 1106-1112. DOI:http://dx.doi.org/10.1016/ j.ajog.2003.09.072.
[37] DISILVESTRO R A, COUSINS R J. Mediation of endotoxininduced changes in zinc metabolism in rats[J]. American Journal of Physiology-Endocrinology and Metabolism, 1984, 247(4): E436-E441.
[38] 邱炳源, 徐乐焱. 锌金属硫蛋白预防甲基汞对细胞膜损伤作用的实验研究[J]. 广东微量元素科学, 1999, 6(2): 15-18.
[39] VALLEE B L, FALCHUK K H. The biochemical basis of zinc physiology[J]. Physiological Reviews, 1993, 73(1): 79-118.
[40] MACKENZIE G G, ZAGO M P, AIMO L, et al. Zinc deficiency in neuronal biology[J]. IUBMB Life, 2007, 59(4/5): 299-307. DOI:10.1080/15216540701225966.
[41] 徐乐焱, 邱炳源, 袁兰, 等. 锌金属硫蛋白抗甲基汞对细胞膜构象的影响[J]. 中国公共卫生学报, 1997, 16(2): 117-118.
[42] BINDER D K, SCHARFMAN H E. Brain-derived neurotrophic factor[J]. Growth Factors, 2004, 22(3): 123-131. DOI:10.1080/089771 90410001723308.
[43] NOSEK K, DENNIS K, ANDRUS B M, et al. Context and straindependent behavioral response to stress[J]. Behavioral and Brain Functions, 2008, 4(23): 1-8. DOI:10.1186/1744-9081-4-23.
[44] CORONA C, MASCIOPINTO F, SILVESTRI E, et al. Dietary zinc supplementation of 3xTg-AD mice increases BDNF levels and prevents cognitive deficits as well as mitochondrial dysfunction[J]. Cell Death & Disease, 2010, 1(10): e91. DOI:10.1038/cddis.2010.73.
[45] MÜLLER H W, JUNGHANS U, KAPPLER J. Astroglial neurotrophic and neurite-promoting factors[J]. Pharmacology & Therapeutics, 1995, 65(1): 1-18. DOI:10.1016/0163-7258(94)00047-7.
[46] CHOWANADISAI W, KELLEHER S L, LÖNNERDAL B. Maternal zinc deficiency reduces NMDA receptor expression in neonatal rat brain, which persists into early adulthood[J]. Journal of Neurochemistry, 2005, 94(2): 510-519. DOI:10.1111/j.1471-4159.2005.03246.
[47] SIDER L H, HUCKE E E T S, FLORIO J C, et al. Influence of time of day on hypothalamic monoaminergic activity in early pregnancy: effect of a previous reproductive experience[J]. Psychoneuroendocrinology, 2003, 28(2): 195-206. DOI:10.1016/S0306-4530(02)00016-1.
Effect and Underlying Mechanism of Prenatal Zinc Treatment on Stress Response in Adult Female Wistar Rat Offspring Exposed to Lipopolysaccharide during Pregnancy
ZUO Manhua
1,2, HUANG Debin
1, HU Xiuying
2, TANG Jun
3,*
(1. Medical College, Hubei University for Nationalities, Enshi 445000, China; 2. West China School of Medicine, Sichuan University, Chengdu 610041, China; 3. Department of Nephrology, the Central Hospital of Enshi Autonomous Prefecture, Enshi 445000, China)
Abstract:Objective: To explore the effects and underlying mechanisms of prenatal zinc treatment (zinc sulfate injection) for pregnant rats exposed to lipopolysaccharide on the behavior and neuroimmune system of their adult female offspring. Methods: Totally 15 Wistar rats were divided into 3 groups randomly and injected with lipopolysaccharide (LPS) plus zinc sulfate (LPS + Zn), LPS plus sterile saline (SAL) (LPS + SAL), and SAL plus SAL (SAL + SAL), respectively. Two or three female offspring during postnatal days 81–86 (n = 10–12 per group) were chosen. The offspring that were in diestrus or metestrus were placed in a restraint stress tube for 2 hours. In the fi nal 5 min of restraint stress, they were subjected to different ultrasonic vocalizations. The behavioral duration times and silence duration times were recorded. After the ultrasonic vocalization test, the rats were removed from the restraint tube and observed in an open fi eld to evaluate motor and anxiety parameters immediately. Immediately after the open fi eld test, trunk blood was collected in conical tubes, and serum corticosterone levels and brain derived neurotropic factor were evaluated by enzyme-linked immunosorbent assay (ELISA). The levels of monoamine and their turnover in the hypothalamus and striatum were determined by high-performance liquidchromatography (HPLC). Results: Longer silence time (P < 0.05, P < 0.01), longer travel distance (P < 0.05, P < 0.001), faster average response speed (P < 0.05, P < 0.001), shorter self-grooming time (P < 0.001, P < 0.01), and lower metabolic rate of norepinephrine in striatum (P < 0.05, P < 0.01) in the LPS + Zn group were observed when compared with the LPS + SAL and SAL + SAL groups. Lower levels of serum corticosterone (P < 0.05) in the LPS + Zn group were also found when compared with the LPS + SAL group. Conclusion: Prenatal zinc treatment has a potential beneficial effect on adult female rat offspring stricken with infectious/inflammatory processes during gestation by reducing the stress response.
Key words:zinc; gestation; rat; lipopolysaccharide (LPS); offspring; stress response
DOI:10.7506/spkx1002-6630-201601032
中图分类号:R363
文献标志码:A
文章编号:1002-6630(2016)01-0182-07
引文格式:
左满花, 黄德斌, 胡秀英, 等. 锌对孕期暴露脂多糖Wistar大鼠雌性成年子代行为的影响及其机制[J]. 食品科学, 2016, 37(1): 182-188. DOI:10.7506/spkx1002-6630-201601032. http://www.spkx.net.cn
ZUO Manhua, HUANG Debin, HU Xiuying, et al. Effect and underlying mechanism of prenatal zinc treatment on stress response in adult female Wistar rat offspring exposed to lipopolysaccharide during pregnancy[J]. Food Science, 2016, 37(1): 182-188. (in Chinese with English abstract) DOI:10.7506/spkx1002-6630-201601032. http://www.spkx.net.cn
收稿日期:2015-03-11
基金项目:国家自然科学基金面上项目(81360654)
作者简介:左满花(1980—),女,讲师,博士研究生,研究方向为微量元素与健康。E-mail:39942922@qq.com
*通信作者:唐俊(1977—),男,主治医师,硕士,研究方向为慢性肾脏病防治。E-mail:mailzuoi80@163.com