
图1 凝乳酶转化过程
Fig.1 Conversion process of chymosin
[3]
杭 锋 1,2,洪 青 2,王钦博 2,刘沛毅 2,刘振民 2,陈 卫 1,*
(1.江南大学食品学院,食品科学与技术国家重点实验室,江苏 无锡 214122;2.乳业生物技术国家重点实验室,光明乳业股份有限公司乳业研究院,上海 200436)
摘 要:传统干酪制作使用的凝乳酶来源于小牛皱胃,目前小牛皱胃酶的供应量仅能满 足世界干酪产量所需凝乳酶的20%~30%。小牛皱胃酶的供需矛盾和价格高昂等因素,使得寻求其替代物成为乳品领域科学研究的热点之一。本文首先介绍了小牛皱胃酶的研究现状,其次从动物、植物、基因重组以及微生物来源,综述了不同凝乳酶之间酶性质差异以及凝乳酶在干酪应用中的研究,旨在为凝乳酶的研究提供一定的理论参考,为寻找凝乳酶替代物提供思路。
关键词:凝乳酶;凝乳酶活力;蛋白水解活力;干酪
凝乳酶(milk-clotting enzymes,MCEs)是制作干酪时凝固牛乳用的酶制剂。MCEs的凝乳性能及蛋白水解能力会最终影响干酪得率、质构和风味。传统干酪加工中所使用的小牛皱胃酶(calf rennet)来源于未断奶小牛第四胃(皱胃)。随着世界干酪产量的不断增加,小牛皱胃酶的供应已出现世界性短缺。为此,国内外学者进行了大量的研究以寻找小牛皱胃酶的替代物,利用微生物生产MCEs是目前最有效的发展途径。
1.1 小牛皱胃酶
早在公元前6世纪,小牛皱胃酶已被应用于干酪制作中,是人类最早用于食品生产的酶之一 [1]。小牛皱胃酶来源于未断奶小牛的皱胃,其中主要有凝乳酶(chymosin,EC3.4.23.4)、胃蛋白酶A(pepsin A,EC 3.4.23.1)和胃亚蛋白酶(gastriscin或pepsin B或pepsin C,EC3.4.23.3) [2],凝乳酶根据第244位氨基酸残基的不同又分为凝乳酶A(残基为Asp)和凝乳酶B(残基为Gly) [3];成年反刍动物皱胃则主要分泌胃蛋白酶A。在最初状态时,凝乳酶是以酶原前体(preprochymosin)的形式分泌至皱胃中,16 个残基组成的酶原前体被去除后转变为凝乳酶原(prochymosin),由365 个氨基酸残基组成,分子质量为40 777 D [4]。在酸性条件下,非活性凝乳酶原被进一步分解成两种活性物质:当pH值达到4.2时,N端42 个氨基酸残基被水解除去,生成由323 个氨基酸残基组成分子质量为35 600 D的凝乳酶(chymosin);在pH值为2.0时,N端27 个氨基酸残基被水解除去,生成由337 个氨基酸残基组成分子质量为37 400 D的假凝乳酶(pseudochymosin),该酶在pH<3.0或pH>6.0时稳定,在pH值为4.5时进一步转变成凝乳酶(图1)。

图1 凝乳酶转化过程
Fig.1 Conversion process of chymosin
[3]
1.2 小牛皱胃酶的凝乳机制
凝乳酶介导凝乳反应涉及两个步骤 [5]:1)酶解酪蛋白(casein,CN):凝乳酶主要水解κ-CN中的Phe 105-Met 106的肽键,生成κ-酪蛋白巨肽(κ-casein macropeptide)和副κ-CN;2)当足够的κ-CN被水解时,副κ-酪蛋白发生聚集形成三维网状凝胶,Ca 2+促发酪蛋白胶束聚集,进而引起酪蛋白胶束失稳并形成干酪凝块(图2)。Hsieh等 [6]利用十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(sodium dodecyl sulfate-polyacrylamide gels electrophoresis,SDS-PAGE)、二维凝胶电泳和质谱研究手段的蛋白质组学方法进一步阐明了凝乳酶的凝乳机理。通常κ-CN的水解度要达到80%~90%时才能发生凝乳,在凝乳第二步非酶反应过程,pH值的降低、温度的升高以及Ca 2+浓度的增加均可加速干酪凝乳过程 [7]。

图2 凝乳酶凝乳机制
[6]
Fig.2 Mechanism for the coagulation of milk proteins by chymosin
[6]
β-LG. β-乳球蛋白(β-lactoglobulin)。
目前,小牛皱胃凝乳酶供应量仅能满足20%~30%世界干酪产量。小牛皱胃凝乳酶的供需矛盾和价格高昂、宗教(伊斯兰教和犹太教)、饮食(素食主义)以及食品法规等因素,使得寻求其替代物成为乳品领域科学研究的热点之一。
小牛皱胃凝乳酶替代物应当具备以下几个特征 [8]:1)在干酪加工中酸性pH值和一定温度条件下具有较高活力,在pH 6.0~6.3条件下形成的凝乳具有更加紧密和相互作用的结构;2)能迅速水解κ-CN和较低的蛋白水解活力,即凝乳酶活力(milk-clotting activity,MCA)/非特异性蛋白水解活力(proteolytic activity,PA)的比值必须高;3)具有较高的热敏性、较低的热处理性来确保乳清中没有残留酶活性,从而提高乳清得率。目前,国内外学者主要从动物、植物、基因重组以及微生物来源进行凝乳酶替代物的相关研究。
2.1 动物来源凝乳酶
2.1.1 小型反刍动物凝乳酶
在地中海沿岸国家,来源于小型反刍动物(小绵羊、小山羊)凝乳酶被广泛用于生产多种原产地保护(protected designation of origin,PDO)干酪,如意大利的Fiore Sardo、Pecorino Romano和Canestrato Pugliese干酪以及希腊的Feta干酪。这种凝乳酶除了具有蛋白水解活力外,还具有使干酪产生特征风味的解脂酶活性。解脂酶主要为胃前脂肪酶(pregastric lipases,PGLs)和胃前酯酶(pregastric esterases,PGEs)。PGEs的解脂作用影响游离 脂肪酸(free fatty acid,FFA)的含量和比例,在Fiore Sardo、Pecorino Romano和Canestrato Pugliese干酪成熟过程中水解脂肪酸生成FFA,最终使得这些干酪呈现辛辣的特征风味 [2,9]。
2.1.2 水牛凝乳酶
与小牛凝乳酶相比,水牛凝乳酶在稳定性和蛋白水解活力等方面有细微差别。Mohanty等 [10]从1~2月龄水牛皱胃组织中分离纯化水牛凝乳酶,其分子质量为35.6 kD,N端前8 个氨基酸组成(甘氨酸-谷氨酸-缬氨酸-丙氨酸-丝氨酸-缬氨酸-脯氨酸-亮氨酸)与小牛凝乳酶氨基酸序列相同。水牛凝乳酶在55 ℃条件下处理15 min能保持相对稳定,60 ℃条件下处理15 min剩余酶活力为50%,较小牛凝乳酶的热稳定性稍高;在pH 5.5时具有最大的凝乳酶活力,随着pH值升高,MCA逐渐降低,pH≥7.0时凝乳酶活性消失;MCA与PA的比例为3.03。
2.1.3 骆驼凝乳酶
骆驼凝乳酶由分子质量为52 kD和39 kD两种组分构成 [11],但通过基因克隆表达的骆驼凝乳酶SDS-PAGE表观分子质量为40 kD [12]。骆驼凝乳酶作用于骆驼乳κ-CN的位点为Phe 97-Ile 98的肽键 [13],作用于牛乳κ-CN的水解位点仍是Phe 105-Met 106的肽键。与小牛凝乳酶相比,骆驼凝乳酶对牛乳具有更强的凝乳作用。在骆驼和小牛凝乳酶水解κ-CN的Michaelis-Menten模型中,与小牛凝乳酶相比,骆驼凝乳酶亲和力(K m)约低30%,但催化速率(k cat)高约60%,导致催化效率(k cat/K m)提高了约15%。骆驼凝乳酶表面较低密度的负电荷簇降低了κ-CN中His-Pro簇的静电引力,进而降低了底物亲和力,加速了酶-底物的分离 [14]。
2.2 植物来源凝乳酶
几乎所有的植物组织均发现具备凝乳功能的蛋白酶 [15],5 种植物内肽酶已得到明确描述,分别为丝氨酸(serine proteases,SPs)、半胱氨酸(cysteine proteases,CPs)、天冬氨酸(aspartic proteases,APs)、苏氨酸(threonine proteases,TPs)和金属蛋白酶(metallo proteases,MPs)。目前由于大部分植物蛋白酶凝乳存在过度蛋白水解特性降低干酪得率和产生苦味问题,在干酪生产中具有一定的局限性 [16]。而部分植物天冬氨酸蛋白酶具有与小牛皱胃酶相似的特性,引起了食品工业的广泛关注 [17]。目前已报道的植物凝乳酶均属于CPs、SPs和APs类型 [15]。
2.2.1 APs型
从罗马时期开始,含有蛋白酶的菜蓟属植物如刺苞菜蓟、蒿草和朝鲜蓟花朵水溶性提取物就已经用于传统绵羊或山羊乳干酪制作中 [18]。刺苞菜蓟花朵中含有能够切断κ-CN中的Phe 105-Met 106肽键并促使凝乳的APs [19],该粗酶可进一步分离纯化,并按其活力分为3 种活性蛋白酶异构体。Sousa等 [20]表明该酶3 种异构体分离图谱中仅峰2和峰3组分具有凝乳和蛋白水解酶活力,这两种蛋白酶后来被命名为cardosin A(峰2)和cardosin B(峰3)。根据其水解特性和活力,cardosin A与凝乳酶相似,cardosin B与胃蛋白酶相似,cardosin B较cardosin A对酪蛋白水解度更高 [21]。刺苞菜蓟来源的凝乳酶由于其对牛乳α s-CN和β-CN水解会形成苦味肽而不用于牛乳干酪的生产中 [19,21]。
2.2.2 CPs型
CPs是分布最广泛的植物蛋白酶,主要来源于植物乳液中。目前,有关于菠萝、牛角瓜、无花果、木瓜、生姜、猕猴桃以及野木瓜等来源CPs蛋白酶具有凝乳作用的报道 [22-26]。由于该类蛋白酶具有较广的温度适宜范围和pH值稳定性,已在食品工业中也得到了广泛应用。CPs的活性中心具有一个CPs残基,导致其最大的缺点是其酶活力易被空气氧和金属离子降低。因此,使用这类酶需要还原剂和金属螯合剂,故而不具有经济性和便利性。
2.2.3 SPs型
SPs活性中心由SPs、组氨酸和Aps这3 种氨基酸残基组成“催化三联体”结构,哺乳动物中的胰蛋白酶、胰凝乳蛋白酶家族及微生物中枯草杆菌蛋白酶家族均属于SPs。已有茄属植物、莴苣、鹊肾树、大戟属和榕属植物来源SPs蛋白酶具有凝乳作用的报告,其中银叶茄成熟浆果在墨西哥部分地区已用于制作Filata干酪Asadero长达数百年之久 [16,27-31]。与CPs蛋白酶不同的是,在高温、高pH值以及存在表面活性剂和氧化剂等严苛的条件下,SPs蛋白酶仍能保持稳定和活性 [22],这一特点在工业化生产中非常有用,但不符合干酪的加工需求。
2.3 基因重组凝乳酶
由于小牛皱胃酶价格昂贵与稀缺等原因,将小牛皱胃酶编码基因在霉菌、食品级酵母和大肠杆菌(Escherichia coli)等宿主中并由lac、trp、trp-beta和gly A基因启动子控制表达来生产重组皱胃酶已成为解决现实问题的有效途径之一。重组凝乳酶通常只含有一种基因变体,而小牛皱胃酶可能含有A、B、C 3 种基因变体外还含有胃蛋白酶 [32]。通过黑曲霉、乳酸克鲁维酵母(Kluyveromyces lactis)和E. coli的基因处理而产生的凝结剂分别称为Chymogen(Genencor/Chr. Hansens)、Maxiren(Gist-brocades/DSM)和Chy-Max(Pfizer),它们在美国和英国被广泛用于干酪生产中,超过50%的干酪已使用重组凝乳酶来生产。但一些国家如法国、德国和荷兰禁止使用重组凝乳酶,动物和微生物仍是干酪生产MCEs的主要来源。
2.4 微生物来源MCEs
许多微生物,尤其是霉菌和细菌来源的胞外蛋白酶具有与MCEs相似的性质,较植物和动物来源的MCEs,微生物来源的MCEs具有生产成本低、更广泛的生化多样性以及简便的基因改造方法。目前,微生物蛋白酶已占据了全球酶市场份额的65%,微生物MCEs更是占到了全球蛋白酶市场份额的33%。然而,微生物MCEs通常具有较高的蛋白水解能力,导致蛋白质降解并转化为乳清,对干酪得率具有负面作用,因此只有部分适于干酪生产 [ 8]。
2.4.1 真菌来源MCEs
真菌来源MCEs与小牛皱胃酶具有相似的凝乳机制,但前者具有对干酪终产品不利的、更高的化学稳定性和较高PA的影响。如嗜热菌米黑根毛霉(Rhizomucor miehei)产生的MCE的MCA/PA比值较小牛皱胃酶低,但PA和耐热性较小牛皱胃酶强,导致了干酪得率降低,55 ℃条件下热处理30 min后残存PA达95%,成熟过程中对酪蛋白进行非特异性水解,造成干酪苦味形成及质构缺陷;随乳清排除的MCE对乳清蛋白也存在过度水解现象,降低了经济利用价值。也有研究发现纯化的真菌MCEs与小牛MCEs混合物较单独的小牛MCEs在干酪成熟和风味形成具有一定的优势。此外,不同菌株产生的MCEs在氨基酸组成、pI、比活力和糖基化特性等方面存在明显差异,从而表征出不同的酶学特性。近年来,已报道的真菌产酶水平及其凝乳酶的酶学特性见表1。
表1 不同真菌的MCEs产量及其酶学特性
Table1 Production and characteristics of milk-clotting enzymes from different fuunnggii
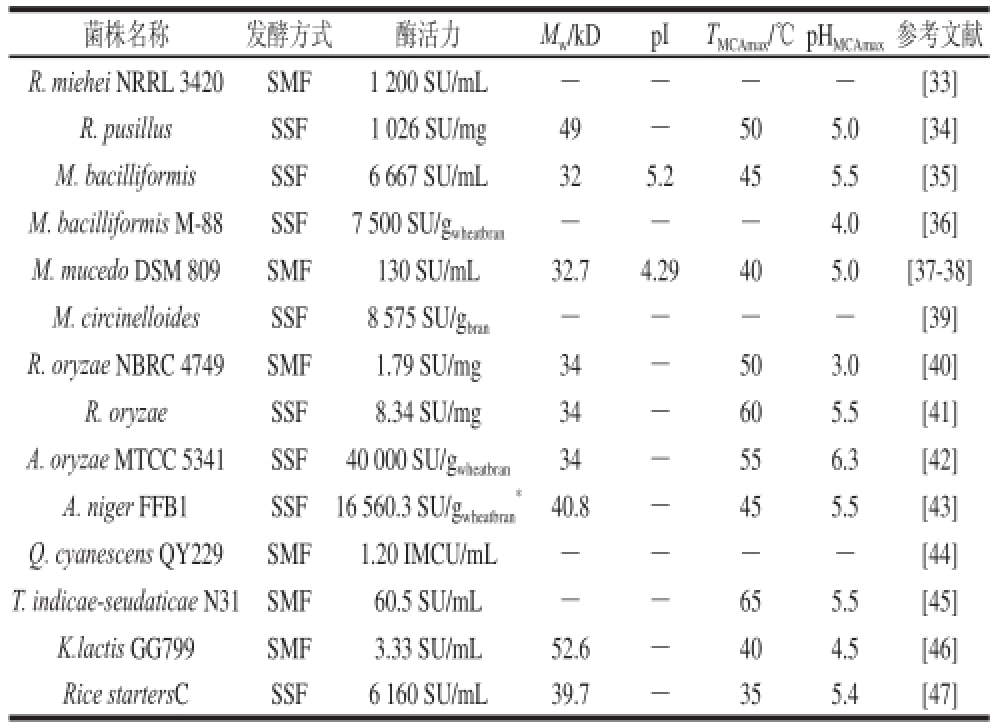
注:SMF.深层发酵(submerged fermentation);SSF.固态发酵(solid state fermentation);-.文献未涉及。下同。
?
2.4.2 细菌来源MCEs
近年 来,有关细菌MCEs的研究报道逐渐增加,相关菌株MCEs产量及其酶学特性见表2。芽孢杆菌(Bacillus spp.)主要在对数生长周期分泌蛋白酶,以中性和碱性蛋白酶居多,并主要属于SPs、CPs和MPs家族,在pH 5.0~8.0较窄的范围内具有活性并且热稳定较低,由于其反应速率中等,中性蛋白酶较动物蛋白酶水解蛋白可产生更少的苦味物质 [48]。
表2 不同细菌MCEs产量及其酶学特性Table 2 Production and characteristics of milk-clotting enzymes from different bacteria

注:*.MCEs酶活力测定方法和定义方法与其他引用文献不同。
?
干酪品质主要由MCEs、乳内源酶以及来源于发酵剂和非发酵微生物酶类引起的。蛋白质水解是凝乳和干酪成熟过程中一个关键的生化反应过程 [59]。κ-CN的非特异水解会影响凝乳速率,进而影响干酪的得率、质构和风味,因此在研究一种潜在MCEs时,评价其对酪蛋白的降解模式至关重要。
3.1 MCEs对干酪组成、理化指标和得率的影响
干酪加工伴随着酪蛋白、脂肪浓缩的脱水过程,因此,脂肪、蛋白回收率及其干酪得率是评判新型MCEs的重要指标 [60]。
将基因重组MCEs和板栗疫病菌(Cryphonectria parasitica)MCEs分别按1∶0、0∶1、67∶33和33∶67比例混合制作Cheddar干酪,发现4 组干酪在制作第1天时理化指标间均无显著差异 [32]。以小牛MCEs和R. miehei NRRL 2034 MCEs制作的白奶酪在60 d成熟过程中较小牛MCEs干酪组在总固形物(total solids,TS)、总氮(total nitrogen,TN)和脂肪含量指标上无显著差异 [61]。以小牛MCEs和解淀粉芽孢杆菌D4 MCEs制作的Cheddar干酪,除了微生物MCEs组在第40天和第60天的pH值显著低于小牛MCEs组干酪外,水分、脂肪、蛋白质、盐含量均无显著差异 [62]。以Thermomucor indicae-seudaticae N31MCEs与R. miehei来源的商业化MCEs制作的半硬质Prato干酪在酸度、水分含量、灰分及盐含量均存在差异,T. indicae-seudaticae N31干酪组的酸度更高、水分含量更低、灰分和盐含量更高 [63];与商业化根毛霉MCEs制作Prato干酪的比较研究中,T. indicae-seudaticae N31 MCEs在凝乳过程中pH值降低程度高于根毛霉MCEs,前者干酪脱水作用更强、水分含量更低,进而导致成熟过程中的蛋白水解程度和pH 4.6可溶性氮含量更低、硬度更高 [61]。小牛皱胃酶、黑曲霉重组MCEs和R. miehei MCEs对白色盐腌制奶酪60 d成熟期间的滴定酸度、干物质和盐含量影响显著,在成熟初期,小牛皱胃酶干酪组在成熟起始阶段滴定酸度值最高、盐含量最低,重组MCEs组的干物质含量最低,主要可能与其在排乳清阶段形成可保留更多水的结构有关 [64]。不同来源MCEs对干酪得率的影响见表3。
表3 不同来源凝乳酶对干酪得率的影响
Table3 Influence of milk-clotting enzymes on cheese yield

微生物MCEs来源干酪得率显著性参考文献对照组(来源)微生物凝乳酶B. amyloliquefaciens D411.03%(小牛MCEs)10.89%P<0.05[62] M. circinelloides163 g/L(小牛MCEs)172 g/L-[39] Mucor J20164 g/L(R. miehei MCEs)160 g/L-[65] R. miehei NRRL 203428.11 g/100 mL(小牛MCEs)29.28 g/100 mL-[66] T. indicae-seudaticae N311 kg/9.9 L(R. miehei MCEs)1 kg/10.5 L-[63] T. indicae-seudaticae N319.57%(R. miehei MCEs)9.48%P>0.05[60]
3.2 MCEs对干酪成熟过程的影响
干酪成熟是一个缓慢、费用较高的过程,因此,加速干酪成熟所带来经济效益十分可观,干酪促熟主要集中在蛋白质水解方面。微生物MCEs蛋白水解能力之间存在差异,对不同酪蛋白的水解能力有所不同,微生物MCEs由于较强的蛋白水解活力表现为对干酪成熟具有促进作用。
如利用小牛MCEs、黑曲霉重组MCEs和R. miehei MCEs制作Braided干酪时发现,R. miehei MCEs制作的干酪在第90天时水溶性氮(water soluble nitrogen,WSN)含量最大,成熟度最高,表明R. miehei MCEs具有较高的水解能力 [67]。R. miehei和C. parasitica来源MCEs对绵羊酪蛋白的水解能力要高于小牛和绵羊凝乳酶,C. parasitica MCEs的蛋白水解程度较R. miehei MCEs更强 [68]。小牛MCEs和R. miehei NRRL 2034 MCEs制作的白干酪在成熟过程中pH值均逐渐下降,可溶性氮(soluble nitrogen,SN)、挥发性脂肪酸(total volatile fatty acids,TVFAs)、酪氨酸(tyrosine,Tyr)和色氨酸(tryptophan,Try)含量均逐渐升高,但微生物MCEs干酪组pH值下降速率更快,SN、TVFAs、Tyr和Try含量更高,这也表明R. miehei NRRL 2034MCEs的蛋白水解和脂解能力更强 [66]。以R. miehei凝乳酶制作的Malatya干酪在成熟过程中WSN较小牛MCEs干酪中更高,α s1-CN水解程度更强,表现为质地较软和融化性较好 [61]。随着成熟期的延长,T. indicae-seudaticae N31和根毛霉制作的Prato干酪(pH 4.6)的可溶性氮和三氯乙酸可溶性氮的含量逐渐升高、硬度逐渐下降,经毛细管电泳,α s1-CN 8P和α s1-CN 9P均在最初的19 d成熟期内开始降解,α s1-CN 8P的降解产物α s1-Ι-CN 8P在成熟的前5 d即可观察到,而α s1-CN 9P降解产物α s1-Ι-CN 9P在成熟33 d可明显观察到,并分别在43 d和33 d完全降解;β-CN也逐渐降解,生成γ-CN和γ 2-CN,其中γ 2-CN也在33 d后可明显观察到 [60]。
3.3 MCEs对干酪质构和风味的影响
干酪成熟过程中的流变学特征变化复杂,主要受α s1-CN、β-CN及其后续更加广泛的蛋白水解影响,α s1-CN的水解程度与干酪融化性成正相关,与干酪硬度成负相关,而β-CN的水解则与干酪融化性成负相关,与硬度成正相关 [32]。干酪成熟过程中蛋白质水解对风味特性也有着极其重要的影响。
将基因重组MCEs和C. parasitica MCEs分别按不同比例混合制作Cheddar干酪发现,随着C. parasitica MCEs比例的增加,Cheddar干酪的融化性和流动性逐渐增强,硬度显著提高;风味、不良风味强度和感官接受度方面无明显差异;咀嚼过程中的苦味、致密度和硬度方面明显增强。以100%重组MCEs制作的干酪酸味和苦味最弱、质地最软 [32]。An Zhigang等 [62]以小牛MCEs和解淀粉芽孢杆菌D4的MCEs制作Cheddar干酪发现,硬度、弹性和内聚性3 个指标均呈下降趋势,微生物MCEs组硬度显著低于小牛MCEs组,而在弹性和内聚性方面无差异;随着成熟时间的增加,两组干酪的储能模量(G’)均呈逐渐下降趋势,反映干酪内部蛋白质间相互作用力的损耗角正切(tanδ)最大值也逐渐下降,两个流变学指标组间无显著差异。
以小牛MCEs和R. miehei NRRL 2034MCEs制作的白奶酪在60 d成熟过程中,由于R. miehei NRRL 2034MCEs的蛋白水解和解脂能力更强,在风味和质构上也表现出该组干酪较小牛MCEs干酪具有更强的风味和较软的质构,但均未观察到风味缺陷和苦味,且微生物MCEs组干酪略高于小牛MCEs组干酪 [66]。以R. miehei MCEs制备的Mozzarella干酪较小牛MCEs和重组MCEs具有最高的硬度,随成熟期延长风味和质构的感官评分而增加,但外观评分则逐渐下降,R. miehei MCEs干酪组风味和质构较其他两组干酪评分更高 [69]。由黑曲霉重组MCEs制备的白色盐腌奶酪在色泽、外观、滋气味感官评分较高,色泽、外观、滋气味和质构方面的综合感官评分非常接近R. miehei MCEs干酪组,且均高于小牛皱胃酶干酪组 [64]。
由于我国原制干酪产业长期以来未形成大规模的工业化生产,在乳品消费市场中份额也较小,因此,关于原制干酪生产加工中所需MCEs等方面的研究一直未受重视。随着我国干酪市场需求增长和加工产业的兴起,必将进一步加剧全球MCEs市场的需求。
本文综述了不同来源的MCEs及其相应的酶学性质。微生物源MCEs因周期短、产量高、生产成本低、经济效益高等优点,是解决小牛皱胃酶短缺的有效途径,筛选高产、高MCA/PA比值MCEs菌株是该领域的重要研究工作。
参考文献:
[1] FOX P, MCSWEENEY P. Rennets: their role in milk coagulation and cheese ripening[M]. London: Springer, 1997: 1-49. DOI:10.1007/978-1-4613-1121-81.
[2] MOSCHOPOULOU E. Characteristics of rennet and other enzymes from small ruminants used in cheese production[J]. Small Ruminant Research, 2011, 101(1/3): 188-195. DOI:10.1016/ j.smallrumres.2011.09.039.
[3] MOHANTY A K, MUKHOPADHYAY U K, GROVER S, et al. Bovine chymosin: production by rDNA technology and application in cheese manufacture[J]. Biotechnology Advances, 1999, 17(2/3): 205-217. DOI:10.1016/S0734-9750(99)00010-5.
[4] KUMAR A, GROVER S, SHARMA J, et al. Chymosin and other milk coagulants: sources and biotechnological interventions[J]. Critical Reviews in Biotechnology, 2010, 30(4): 243-258. DOI:10.3109/07388 551.2010.483459.
[5] BRUNO M A, LAZZA C M, ERRASTI M E, et al. Milk clotting and proteolytic activity of an enzyme preparation from Bromelia hieronymi fruits[J]. LWT-Food Science and Technology, 2010, 43(4): 695-701. DOI:10.1016/j.lwt.2009.12.003.
[6] HSIEH J F, PAN P H. Proteomic profiling of the coagulation of milk proteins induced by chymosin[J]. Journal of Agricultural and Food Chemistry, 2012, 60(8): 2039-2045. DOI:10.1021/jf204582g.
[7] NAJERA A I, de RENOBALES M, BARRON L J R. Effects of pH, temperature, CaCl 2and enzyme concentrations on the rennet-clotting properties of milk: a multifactor ial study[J]. Food Chemistry, 2003, 80(3): 345-352. DOI:10.1016/S0308-8146(02)00270-4.
[8] JUNIOR B R D C L, TRIBSTA A L, CRISTIANINI M. High pres sure homogenization of porcine pepsin protease: effects on enzyme activity, stability, milk coagulation profile and gel development[J]. PLoS ONE, 2015. DOI:10.1371/journal.pone.0125061.
[9 ] ADDIS M, PIREDDA G, PIRISI A. The use of lamb rennet paste in traditional sheep milk cheese production[J]. Small Ruminant Research, 2008, 79(1): 2-10. DOI:10.1016/j.smallrumres.2008.07.002.
[10] MOHANTY A K, MUKHOPADHYAY U K, KAUSHIK J K, et al. Isolation, p urification and characterization of chymosin from riverine buffalo (Bubalus bubalis)[J]. Journal of Dairy Research, 2003, 70(1): 37-43. DOI:10.1017/S0022029902005927.
[11] ELAGAMY E I. Physicochemical, molecular and immu nological characterization of camel calf rennet: a comparison with buffalo rennet[J]. Journal of Dairy Research, 2000, 67(1): 73-81. DOI:10.1017/ S0022029999003957.
[12] KAPPELER S R, va n den BRINK H J M, RAHBE K-NIELSEN H, et al. Characterization of recombinant camel chymosin reveals superior properties for the coagulation of bovine and camel milk[J]. Biochemical and Biophysical Research Communications, 2006, 342(2): 647-654 . DOI:10.1016/j.bbrc.2006.02.014.
[13] BENKERROUM N, DEHHAOUI M, FAYQ A E, et al. The effect of concentration of chymosin on the yield and sensory properties of camel cheese and on its microbiological quality[J]. International Journal of Dairy Technolog y, 2011, 64(2): 232-239. DOI:10.1111/ j.1471-0307.2010.00662.x.
[14] M±LLER LLER M K K, RATTRAY F P, S± RENSEN J C, et al. Comparison of the hydrolysis of bovine κ-casein by camel and bovine chymosin: a kinetic and specificity stud y[J]. Journal of Agricultural and Food Chemistry, 2012, 60(21): 5454-5460. DOI:10.1021/ jf300557d.
[15] SHAH M A, MIR S A, PARAY M A. Plant proteases as milk-clotting enzymes in cheesemaking: a review[J]. Dairy Scienc e & Technology, 2014, 94(1): 5-16. DOI:10.1007/s13594-013-0144-3.
[16] PIERO A R L, LVANA P, GOFFREDO P. Characterization of“lettucine”, a serine-like protease from Lactuca sativa lea ves, as a nov el enzyme for milk clotting[J]. Journal of Agricultural and Food Chemistry, 2002, 50(8): 2439-2443. DOI:10.1021/jf011269k.
[17] GONZÁLEZ-RÁBADE N, BADILLO-CORONA J A, ARANDABARRADAS J S, et al. Productio n of plant proteases in vivo and in vitro: a review[J]. Biot echnology Advances, 2011, 29(6): 983-996. DOI:10.1016/j.biotechadv.2011.08.017.
[18] REIS P C M, LOUREN O P L, DOMINGOS A, et al. Applicability of extracts from Centaurea calcitrapa in ripening of bovine cheese[J]. International Dairy Jou rnal, 2000, 10(11): 775-780. DOI:10.1016/ S 0958-6946(00)00105-9.
[19] VERISSIMO P, ESTEVES C, FARO C, et al. The vegetable rennet of Cynara cardunculus L. contains two proteinases with chymosin and pepsin-like specificities[J]. Biotechnology Letters, 1995, 17(6): 621-626. DOI:10.10 07/BF00129389.
[20] SOUSA M J, MALCATA F X. Advances i n the role of a plant coagulant (Cynara cardunculus) in vitro and during ripening of cheeses from several milk species[J]. Le Lait, 2002, 82(2): 151-170. DOI:10.1051/lait:2002001.
[21] BARROS R, MALCATA F. Molecular characterization of peptid es released from β-lactoglobulin and α-lactalbumin via cardosins A and B[J]. Journal of Dairy Science, 2006, 89(2): 483-494. DOI:10.3168/jds. S0022-0302(06)72111-7.
[22] TOMAR R, KUMAR R, J AGANNADHAM M. A stable serine protease, wrightin, from the latex of the plant Wrightia tinctoria (Roxb.) R. Br.: purification and biochemical properties[J]. Journal of Agricultural and Food Chemistry, 2008, 56(4): 1479-1487. DOI:10.1021/jf0726536.
[23] HASHIM M M, DONG M, IQBAL M F, et al . Ginger protease used as coagulant enhances the proteolysis and sensory quality of Peshawari cheese compared to calf rennet[J]. Dairy Science & Technology, 2011, 91(4): 431-440. DOI:10.1007/s13594- 011-0021-x.
[24] HUANG X, CHEN L, LUO Y, et al. Purification, characterization, and milk coagulating properties of ginger proteases[J]. Journal of Dairy Science, 2011, 94(5): 225 9-2269. DOI:10.3168/jds.2010-4024.
[25 ] PIERO A R L, PUGLISI I, PETRONE G. Characterization of the purified actinidin as a plant coagulant of bovine milk[J]. European Food Research and Technology, 2011, 233(3): 517-524. DOI:10.1007/ s00217-011-1543-4.
[26] DUARTE A R, DUARTE D M R, MOREIRA K A, et al. Jacaratia corumbensis O. Kuntze a new vegetable source for milk-clotting enzymes[J]. Brazilian Archives of Biology and Technology, 2009, 52(1): 1-9. DOI:10.1590/S1516-891320090001 00001.
[27] NÉSTOR G M, RUBÍ C G D, HÉCTOR J C. Exploring the milk-clotting properties of a plant coagulant from the berries of S.elaeagnifolium var. Cavanilles[J]. Journal of Food Science, 2012, 77(1): C89-C94. DOI:10.1111/j.1750 -3841.2011.02468.x.
[28] TRIPATHI P, TOMAR R, JAGANNADHAM M V. Purification and biochemical characterisation of a novel protease streblin[J]. Food Chemistry, 2011, 125(3): 1005-1012. DOI:10.1016/ j.foodchem.2010.09.108.
[29] YADAV R P, PATEL A K, JAGANNADHAM M. Neriifolin S, a dimeric serine protease from Euphorbia neriifolia Linn.: purification and biochemical characterisation[J]. Food Chemistry, 2012, 132(3): 1296-1304. DOI:10.1016/j.foodchem.2011.11.107.
[30] KUMARI M, SHARMA A, JAGANNADHAM M. Religiosin B, a milk -clotting serine protease from Ficus religiosa[J]. Food Chemistry, 2012, 131(4): 1295-1303. DOI:10.1016/j.foodchem.2011.09.122.
[31] SHARMA A, KUMARI M, JAGANNADHAM M. Religiosin C, a cucumisin-like serine protease from Ficus religiosa[J]. Process Biochemistry, 2012, 47(6): 914-921. DOI:10.1016/ j.procbio.2012.02.015.
[32] KIM S Y, GUNASEKARAN S, OLSON N F. Combined use of chymosin and protease from Cryphonectria parasitica for c ontrol of meltability and firmness of Cheddar cheese[J]. Journal of Dairy Science, 2004, 87(2): 274-283. DOI:10.3168/jds.S0022-0302(04)73166-5.
[33] de LIMA C, CORTEZI M, LOVAGLIO R B, et al. Production of rennet in submerged ferm entation with the filamentous fungus Mucor miehei NRRL 3420[J]. World Applied Sciences Journal, 2008, 4(4): 578-585.
[34] NOUANI A, BELHAMICHE N, SLAMANI R, et al. Extracellular protease from Mucor pusillus: purification and characterization[J]. International Journal of Dairy Technology, 2009, 62(1): 112-117. DOI:10.1111/j.1471-0307.2008.00454.x.
[35] VENERA G D, MACHALINSKI C, ZUMARRAGA H, et al. Further characterization and kinetic parame ter determination of a milk-clotting protease from Mucor bacilliformis[J]. Applied Biochemistry and Biotechnology, 1997, 68(3): 207-216. DOI:10.1007/BF02785991.
[36] FERNÁNDEZ L H, GALLEGO D M V, CASCONE O, et al. Solid state production of a Mucor bacilliformis acid protease[J]. Revista Argentina de Microbiologia, 1996, 29(1): 1-6.
[37] YEGIN S, FERNANDEZ-LAHORE M, GUVENC U, et al. Production of extracellular aspartic proteas e in submerged fermentation with Mucor mucedo DSM 80 9[J]. African Journal of Biotechnology, 2010, 9(38): 6380-6386.
[38] YEGIN S, GOKSUNGUR Y, FERNANDEZ-LAHORE M. Purification, structural characterization, and technological properties of an aspartyl proteinase from submerged cultures of Mucor mucedo DSM 809[J]. Food Chemistry, 2012, 133(4): 1312-1319. DOI:10.1016/ j.foodchem.2012.01.075.
[39] SATHYA R, PRADEEP B, ANGAYARKANNI J, et al. Production of milk clotting protease by a local isolate of Mucor circinelloides under SSF using agro-industrial wastes[J]. Biotechnology and Bioprocess Engineering, 2009, 14(6): 788-794. DOI:10.1007/s12257-008-0304-0.
[40] CHEN C C, CHO Y C, LAI C C, et al. Purification and characterization of a new rhizopuspepsin from Rhizopus oryzae NBRC 4749[J]. Journal of Agricultural and Food Chemistry, 2009, 57(15): 6742-6747. DOI:10.1021/jf8040337.
[41] KUMAR S, SHARMA N S, SAHARAN M R, et al. Extracellular acid protease from Rhizopus oryzae: purification and characterization[J]. Process Biochemistry, 2005, 40(5): 1701-1705. DOI:10.1016/ j.procbio.2004.06.047.
[42] VISHWANATHA K S, APPU R A G, SINGH S A. Production and characterization of a milk-clotting enzyme from Aspergillus oryzae MTCC 5341[J]. Applied Microbiology and Biotechnology, 2010, 85(6): 1849-1859. DOI:10.1007/s00253-009-2197-z.
[43] FAZOUANE-NAIMI F, MECHAKRA A, ABDELLAOUI R, et al. Characterization and cheese-making properties of rennet-like enzyme produced by a local Algerian isolate of Aspergillus niger[J]. Food Biotechnology, 2010, 24(3): 258-269. DOI:10.1080/08905436.2010.507149.
[44] ZHANG Z G, WANG C Z, YAO Z Y, et al. Isolation and identification of a fungal strain QY229 producing milk-clotting enzyme[J]. European Food Research and Technology, 2011, 232(5): 861-866. DOI:10.1007/ s00217-011-1454-4.
[45] SILVA B, GERALDES F, MURARI C, et al. Production and characterization of a milk-clotting protease produced in submerged fermentation by the thermophilic fungus Thermomucor indicaeseudaticae N31[J]. Applied Biochemistry and Biotechnology, 2014, 172(4): 1999-2011. DOI:10.1007/s12010-013-0655-7.
[46] ALMEIDA C M, GOMES D, FARO C, et al. Engineering a cardosin B-derived rennet for sheep and goat cheese manufacture[J]. Applied Microbiology & Biotechnology, 2015, 99(1): 269-281. DOI:10.1007/ s00253-014-5902-5.
[47] ZHAO X, WANG J, ZHENG Z, et al. Production of a milk-clotting enzyme by glutinous rice fermentation and partial characterization of the enzyme[J]. Journal of Food Biochemistry, 2015, 39(1): 70-79. DOI:10.1111/jfbc.12108.
[48] SINGH T, DRAKE M, CADWALLADER K. Flavor of Cheddar cheese: a chemical and sensory perspective[J]. Comprehensive Reviews in Food Science and Food Safety, 2003, 2(4): 166-189. DOI:10.1111/j.1541-4337.2003.tb00021.x.
[49] HE X L, REN F Z, GUO H Y, et al. Purification and properties of a milk-clotting enzyme produced by Bacillus amyloliquefaciens D4[J]. Korean Journal of Chemical Engineering, 2011, 28(1): 203-208. DOI:10.1007/s11814-010-0347-8.
[50] HE X L, ZHANG W B, REN F Z, et al. Screening fermentation parameters of the milk-clotting enzyme produced by newly isolated Bacillus amyloliquefaciens D4 from the Tibetan Plateau in China[J]. Annals of Microbiology, 2012, 62(1): 357-365. DOI:10.1007/s13213-011-0270-1.
[51] DING Z Y, WANG W F, WANG B D, et al. Production and characterization of milk-clotting enzyme from Bacillus amyloliquefaciens JNU002 by submerged fermentation[J]. European Food Research and Technology, 2012, 234(3): 415-421. DOI:10.1007/ s00217-011-1650-2.
[52] DING Z Y, AI L Z, OUYANG A, et al. A two-stage oxygen supply control strategy for enhancing milk-clotting enzyme production by Bacillus amyloliquefaciens[J]. European Food Research and Technology, 2012, 234(6): 1043-1048. DOI:10.1007/s00217-012-1723-x.
[53] SHIEH C J, PHAN THI L A, SHIH I L. Milk-clotting enzymes produced by culture of Bacillus subtilis natto[J]. Biochemical Engineering Journal, 2009, 43(1): 85-91. DOI:10.1016/ j.bej.2008.09.003.
[54] DING Z Y, LIU S P, GU Z H, et al. Production of milk-clotting enzyme by Bacillus subtilis B1 from wheat bran[J]. African Journal of Biotechnology, 2011, 10(46): 9370-9378. DOI:10.5897/AJB10.1647.
[55] LI Y, LIANG S, ZHI D J, et al. Purification and characterization of Bacillus subtilis milk-clotting enzyme from Tibet Plateau and its potential use in yak dairy industry[J]. European Food Research and Technology, 2012, 234(4): 733-741. DOI:10.1007/s00217-012-1663-5.
[56] EL-BENDARY M A, MOHARAM M E, ALI T H. Purification and characterization of milk clotting enzyme produced by Bacillus sphaericus[J]. Journal of Applied Sciences Research, 2007, 3(8): 695-699.
[57] AGRAHARI S, WADHWA N. Isolation and characterization of feather degrading enzymes from Bacillus megaterium SN1 isolated from Ghazipur poultry waste site[J]. Applied Biochemistry and Microbiology, 2012, 48(2): 175-181. DOI:10.1134/ S0003683812020020.
[58] AGEITOS J, VALLEJO J, SESTELO A, et al. Purification and characterization of a milk-clotting protease from Bacillus licheniformis strain USC13[J]. Journal of Applied Microbiology, 2007, 103(6): 2205-2213. DOI:10.1111/j.1365-2672.2007.03460.x.
[59] AZARNIA S, ROBERT N, LEE B. Biotechnological methods to accelerate Cheddar cheese ripening[J]. Critical Reviews in Biotechnology, 2006, 26(3): 121-143. DOI:10.1080/07388550600840525.
[60] ALVES L, MERHEB-DINI C, GOMES E, et al. Yield, changes in proteolysis, and sensory quality of Prato cheese produced with different coagulants[J]. Journal of Dairy Science, 2013, 96(12): 7490-7499. DOI:10.3168/jds.2013-7119.
[61] HAYALOGLU A, KARATEKIN B, GURKAN H. T hermal stability of chymosin or microbial coagulant in the manufacture of Malatya, a Halloumi type cheese: proteolysis, microstructure and functional properties[J]. International Dairy Journal, 2014, 38(2): 136-144. DOI:10.1016/j.idairyj.2014.04.001.
[62] AN Z G, HE X L, GAO W D, et al. Characteristics of miniature Cheddartype cheese made by microbial rennet from Bacillus amyloliquefaciens: a comparison with commercial calf rennet[J]. Journal of Food Science, 2014, 79(2): 214-221. DOI:10.1111/1750-3841.12340.
[63] MERHEB-DINI C, GARCIA G A C, PENNA A L B, et al. Use of a new milk-clotting protease from Thermomucor indicaeseudaticae N31 as coagulant and changes during ripening of Prato cheese[J]. Food Chemistry, 2012, 130(4): 859-865. DOI:10.1016/ j.foodchem.2011.07.105.
[64] ÇEPOĞLU F, GÜLER-AKþN M B. Eff ects of coagulating enzyme types (commercial calf rennet, Aspergillus niger var. awamori as recombinant chymosin and rhizomucor miehei as microbial rennet) on the chemical and sensory characteristics of white pickled cheese[J]. African Journal of Biotechnology, 2013, 12(37): 5588-5594. DOI:10.5897/AJB2013.12912.
[65] TUBESHA Z, AL-DELAIMY K. Ren nin-like milk coagulant enzyme produced by a local isolate of Mucor[J]. International Journal of Dairy Technology, 2003, 56(4): 237-241. DOI:10.1046/j.1471-0307.2003.00113.x.
[66] ABBAS H M, FODA M S, KASSEM J M, et al. Pro duction of white soft cheese using fungal coagulant produced by solid state fermentation technique[J]. World Applied Sciences Journal, 2013, 25(6): 939-944. DOI:10.5829/idosi.wasj.2013.25.06.13385.
[67] ÇELEBI M, ŞIMŞEK B. Effec ts of different coagulant enzymes used on quality of traditional Örgü cheese (Braided cheese)[J]. Mljekarstvo, 2015, 65(1): 57-65. DOI:10.15567/mljekarstvo.2015.0108.
[68] TRUJILLO A J, GUAMIS B, LAENCINA J, et al. Proteolyt ic activities of some milk clotting enzymes on ovine casein[J]. Food Chemistry, 2000, 71(4): 449-457. DOI:10.1016/S0308-8146(00)00170-9.
[69] AHMED N S, EL-GAWAD M, EL-ABD M M, et al. Properti e s of buffalo Mozzarella cheese as affected by type of coagulante[J]. Acta Scientiarum Polonorum Technologia Alimentaria, 2011, 10(3): 339-357.
Advances in Research on Milk-Clotting Enzymes
HANG Feng
1,2, HONG Qing
2, WANG Qinbo
2, LIU Peiyi
2, LIU Zhenmin
2, CHEN Wei
1,*
(1. State Key Laboratory of Food Science and Technology, School of Food Science and Technology, Jiangnan University, Wuxi 214122, China; 2. State Key Laboratory of Dairy Biotechnology, Dairy Research Institute, Bright Dairy and Food Co. Ltd., Shanghai 200436, China)
Abstract:Calf rennet is conventionally used as milk coagulant for the production of cheese. However, the supply of calf rennet is not equivalent to the demand in cheese industry, which merely meets 20%–30% of the global demand for cheese production. Due to the scarcity and high price of calf rennet, it is necessary and urgent to find potential substitutes. This review gives an overview of the current discoveries of calf rennet, the characteristics of different types of milkclotting enzymes including animal rennet, recombinant chymosin, plant- and microbial-derived coagulants, and specifies the application of coagulants in cheese production. The objective of this review is to provide the fundamental theory and inspiring ideas for the researchers and manufacturers to find calf rennet substitutes.
Key words:chymosin; milk-clotting activity; proteolytic activity; cheese
DOI:10.7506/spkx1002-6630-201603047
中图分类号:TS252.1
文献标志码:A
文章编号:1002-6630(2016)03-0273-07
引文格式:
杭锋, 洪青, 王钦博, 等. 凝乳酶的研究进展[J]. 食品科学, 2016, 37(3): 273-279. DOI:10.7506/spkx1002-6630-201603047. http://www.spkx.net.cn
HANG Feng, HONG Qing, WANG Qinbo, et al. Advances in research on milk-clotting enzymes[J]. Food Science, 2016, 37(3): 273-279. (in Chinese with English abstract) DOI:10.7506/spkx1002-6630-201603047. http://www.spkx.net.cn
收稿日期:2015-07-20
基金项目:上海市科委青年科技启明星人才计划项目(14QB1400200);“十二五”国家科技支撑计划项目(2013BAD18B02)
作者简介:杭锋(1982—),男,高级工程师,博士研究生,研究方向为乳品科学与技术。E-mail:fhang0427@126.com
*通信作者:陈卫(1966—),男,教授,博士,研究方向为食品生物技术。E-mail:chenwei66@jiangnan.edu.cn