表1 近5 a报道产EPS的真菌
Table 1 Exopolysaccharide production by fungi reported in last five years

注:WDA.麦提取液葡萄糖琼脂培养基(wheat extract dextrose agar);YPD.酵母提取物蛋白胨葡萄糖培养基(yeast extract peptone dextrose);―.所引文献未作说明。下同。
杨同香1,吴孔阳2,陈俊亮1,唐浩国1,康怀彬1
(1.河南科技大学食品与生物工程学院,河南 洛阳 471023;2.洛阳师范学院生命科学学院,河南 洛阳 471022)
摘 要:真菌胞外多糖具有高黏性、抗氧化、抗病毒和抗肿瘤等多种生物学活性,在食品和医药工业等领域应用广泛。本文结合国内外最新研究成果,综述了近年来有关产胞外多糖的真菌、影响真菌胞外多糖产量的因素、产胞外多糖的菌株的选育策略以及真菌胞外多糖的功能研究,以期为真菌发酵胞外多糖的研究和应用提供参考。
关键词:真菌胞外多糖;产量;发酵;功能
引文格式:
杨同香,吴孔阳,陈俊亮,等.真菌发酵胞外多糖的研究进展[J].食品科学,2016,37(5):265-270.DOI:10.7506/spkx1002-6630-201605046.http://www.spkx.net.cn
YANG Tongxiang,WU Kongyang,CHEN Junliang,et al.Recent advances in fungal exopolysaccharide fermentation[J].Food Science,2016,37(5):265-270.(in Chinese with English abstract)DOI:10.7506/spkx1002-6630-201605046.http://www.spkx.net.cn
多糖在自然界分布极其广泛,植物、动物和微生物体内均有存在,胞外多糖(exopolysaccharide,EPS)通常指微生物在生长代谢过程中分泌到细胞外的一类多糖[1-3]。由于微生物EPS具有多元化的多糖结构和不同的流变学特性,在生物技术领域应用前景十分广阔[4]。近年来,研究人员对微生物EPS开展了广泛的研究,主要包括菌株的分离和鉴定、产EPS菌株选育、EPS生物合成途径、工程菌构建、发酵条件的优化、EPS的分离、纯化、结构鉴定及其生物学活性的研究等,尤其是对细菌EPS的研究进行了较全面的归纳和总结[4-7],而单就对真菌发酵EPS的研究概述则相对较少。另外,如何获得高产EPS产生菌以及如何提高EPS产量,也是研究人员一直以来关心的问题。因此,本课题组结合自己的研究工作,从EPS产生菌、影响真菌EPS产量的因素、产EPS的菌株的选育策略等方面对真菌发酵EPS的研究做一简要概述。
目前公开报道产EPS的真菌至少有上百种[8]。以往研究人员主要从陆地(包括极地环境)采样并分离产EPS的真菌,近些年发现海洋蕴藏着数量丰富且能产生结构新颖EPS的真菌[9]。本文将近5 a有关产EPS真菌的文献进行归纳,见表1。大多数研究者选择马铃薯葡萄糖琼脂培养基(potato dextrose agar,PDA)对产EPS真菌的培养和分离。由于不同真菌生长特性差异,培养温度一般在18~30 ℃。所产EPS的结构也因菌株种属差异而种类繁多,通常发现的真菌EPS以杂多糖居多,当然也有关于同多糖的报道,如Mukhopadhyay等[10]鉴定了一株产EPS的南极土壤嗜冷真菌Thelebolus sp.IITKGP-BT12,进一步研究发现该EPS为葡聚糖,且在基础盐培养基中,多糖产量高达1.94 g/L。
表1 近5 a报道产EPS的真菌
Table 1 Exopolysaccharide production by fungi reported in last five years

注:WDA.麦提取液葡萄糖琼脂培养基(wheat extract dextrose agar);YPD.酵母提取物蛋白胨葡萄糖培养基(yeast extract peptone dextrose);―.所引文献未作说明。下同。
研究发现EPS的生物合成是在细胞内进行,然后分泌到细胞外。而这一过程通常受到诸多因素影响,其中对EPS产量的影响主要受到真菌类型、培养基组成以及发酵条件等因素。
2.1真菌类型
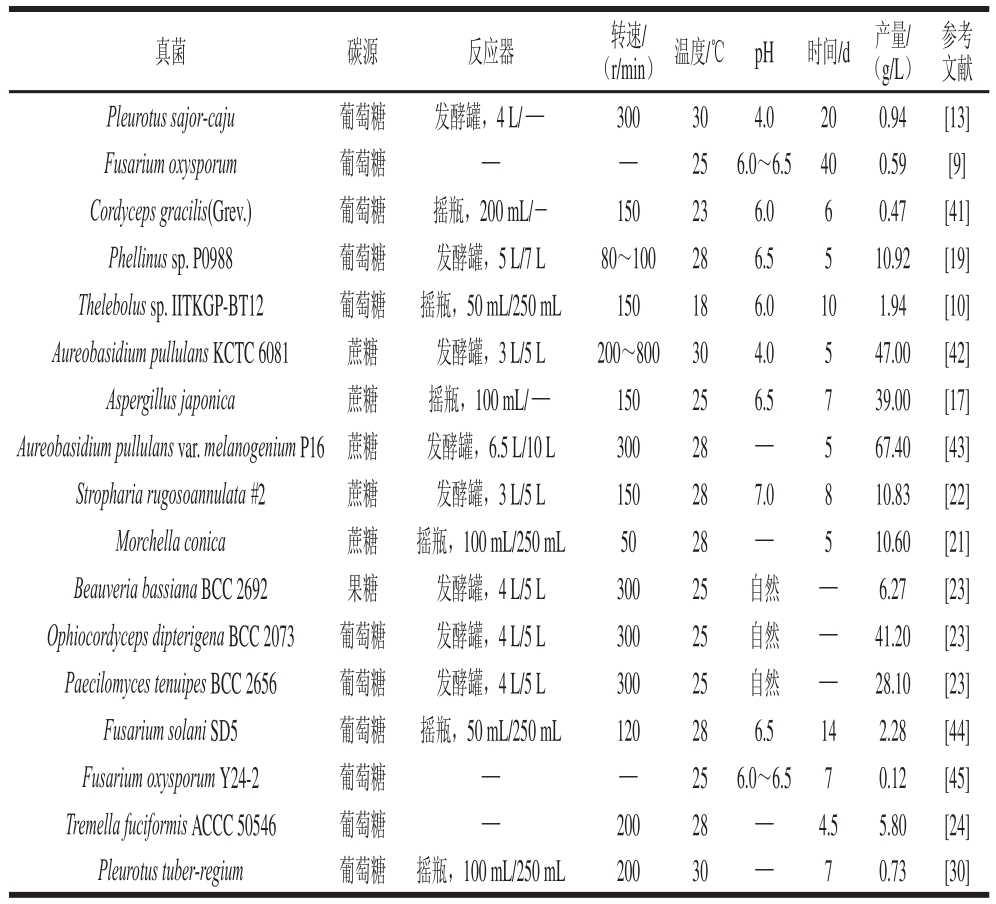
对于未研究的和未开发的真菌,不在统计范围之类,从公开报道的真菌发酵EPS水平来看,担子菌通常比丝状真菌和酵母菌EPS的产量要高[8]。本文对近年来真菌发酵EPS的文献进行归纳(表2),从表2中可以基本了解不同真菌发酵产EPS的能力。
表2 国内外部分真菌EPSS产量
Table 2 Overseas studies on the production of fungal exopolysaccharide
2.2培养基组成
研究表明培养基组成对EPS产量的影响至关重要。微生物发酵EPS,对碳源选择具有一定的偏好性,细菌通常利用蔗糖发酵EPS[32],真菌则通常利用葡萄糖发酵EPS(表2),并且在该条件下可以促进菌丝生长和获得较多的多糖。对于氮源而言,多数真菌发酵EPS,会考虑使用酵母粉[33],有些会辅以脱脂牛奶作为氮源[34]。不过对于工业化发酵生产EPS,最好还要考虑到原料的价格,Sharma等[35]对A.pullulans RBF 4A3利用5 种不同的农副产品作为营养来源发酵生产普鲁兰多糖进行研究,结果发现添加有玉米浆和葡萄糖的培养基,菌株产多糖水平上升,而且经过单因素条件优化后,普鲁兰多糖的产量提高了14%,质量浓度达到88.59 g/L。相关研究结果表明,磷酸盐对于真菌发酵EPS这一过程非常重要,其中磷酸二氢钾和磷酸氢二钾被认为是最为有效的磷源补充剂,而对于其他的一些无机盐,硫酸镁则被认为对真菌发酵EPS有很好促进作用[8]。另外,在真菌发酵EPS过程中,添加某些因子也可以提高真菌发酵EPS水平。这些因子包括植物油、脂肪酸、表面活性剂、核苷酸和维生素等。比如Sheng Long等[36]研究了尿嘧啶对Aureobasidium pullulans CGMCC1234发酵多糖的影响,当菌株发酵48 h时,向培养基中添加5 mmol/L的尿嘧啶,结果发现与不加尿嘧啶的对照组相比,Aureobasidium pullulans CGMCC1234普鲁兰多糖质量浓度达到49.07 g/L,提高了30%。Sheng Long等[37]发现在培养基中加入体积分数为0.5%的吐温-80可以显著提高Aureobasidium pullulans CGMCC1234普鲁兰多糖的产量。事实上,其他研究结果也表明吐温-80能够促进对多种真菌EPS的合成,并从蛋白质组学角度去阐述吐温-80的作用机制[38-39]。
培养基的组分可以直接影响菌体的生长状况,菌体生长状况与EPS产量是否有关联,尚无统一的论证。培养基中的某些成分尽管能够促进菌体生长,但会减少EPS产量。Wu Shengjun等[25]研究发现来源于Aureobasidium pullulans CJ001所产的普鲁兰多糖,与细胞生物量没有相关性,而这与某些细菌EPS产量与生物量相关的结论不一致[40],这有助于研究人员更好地认识菌株类型和培养基差异对真菌发酵EPS的影响。
2.3发酵条件
菌株深层培养技术在商业上被认为是真菌发酵多糖十分有效的方法,且未出现显著的污染问题[46]。大量研究表明真菌发酵EPS需要在好氧条件下完成,溶氧限制将影响EPS产量。Ruperez等[47]通过静息培养和深层培养两种方式比较了Aspergillus parasiticus发酵EPS的能力,结果发现深层培养条件下所得到的EPS产量是静息培养条件下的2.3 倍。目前有关真菌发酵EPS条件研究,多集中于对发酵培养基和发酵产EPS条件的优化,所涉及的实验方法采用常规的统计方法,包括单因素试验、正交试验、部分因子试验以及中心组合试验等优化EPS产量[23,41,44]。不同于细菌生长周期快,真菌发酵EPS通常需要较长的时间,一般需要1 周左右,多的甚至需要发酵20 d(表2)。
获得高产EPS的菌株是发酵优化的前提。对于真核微生物而言,当传统微生物育种技术难以对某些特定菌株的产多糖水平有较大提高时,有必要开发新的菌种选育技术。当然,研究人员需要根据菌株自身特性及实验室条件来设计合理的选育路线。通常情况下,可以在传统育种技术基础上,通过改良或引入新开发的高效微生物进化育种技术获得高产EPS突变株。比如Liu Bin等[48]采用常温常压等离子体技术对海洋Crypthecodinium cohnii进行突变选育,获得一株高产EPS突变子M7,EPS产量达到1.02 g/L,较出发菌株提高了33.85%。DNA改组技术也可获得高产EPS的突变株,Kang Jianxiong等[49]通过在紫外/甲基磺酸乙酯诱变基础上,通过DNA改组技术获得Aureobasidium pullulans突变株F3-2,EPS产量较野生型菌株提高了168%,极大地提高了EPS产量。另外通过控制菌株生长的外部环境,可以在不同程度上提高真菌发酵EPS的产量,比如限制氮源种类和添加量,不仅可以减少投入成本,还可以极大促进EPS的合成[50]。
由表1可知,不同真菌所产EPS具有多样性的结构类型,因其结构的独特性,使得真菌EPS具有丰富的生物学功能和极其广泛的工业应用价值[51]。
4.1抗肿瘤及免疫调节活性
活性真菌多糖通常被描述为生物反应调节剂,主要是基于它们能够触发免疫系统针对癌细胞的非特异性反应[21,52]。Ikekawa[53]通过小鼠喂养真姬菇实验发现,实验组小鼠患癌比例(3/36)远小于对照组(21/36),并认为抑制肿瘤的机制由于免疫增强作用所致,Wasser[54]也认同这一观点,认为食用一些大型真菌可以预防肿瘤的发生和转移。事实上,对于癌症的预防和治疗,从临床实践中发现,大型真菌EPS与化学疗法、外科手术结合起来被认为是非常有效的方法。在动物模型和人类临床实践中,香菇多糖的研究最为透彻,在化学疗法治疗之前,通过给患癌病人注射香菇多糖,可以很好地改善治疗效果[54]。最新研究发现,翅鳞伞菌株(Pholiota squarrosa Quel.)AS5.245 水溶性胞外多糖(water-soluble exopolysaccharide,PEPS)-1对植入小鼠Heps肝癌细胞具有抗肿瘤活性,并猜测这种特性很可能是PEPS-1通过刺激宿主的免疫应答反应实现,但是PEPS-1的结构与功能的关系以及分子作用机制还需进一步研究[11]。除此之外,Aureobasidium pullulans所产的普鲁兰多糖及其各种衍生物也成为研究人员用于治疗肝、肺、脑、脾等相关肿瘤疾病治疗的潜在药物[55-56]。比如Li Huanan等[57]通过合成普鲁兰多糖衍生物(普鲁兰多糖-多柔比星结合物),研究该物质对人肝癌细胞的影响,并认为该结合物有望成为靶向药物载体安全用于给药体系。
4.2抗炎症及抗菌作用
大型真菌所产的一些多糖,其治疗效果已得公认,比如凤尾菇EPS具有镇痛和抗炎作用[13]。Smiderle等[58]从Pleurotus pulmonarius中分离出一种3-O-甲基-半乳甘露聚糖,发现该多糖对醋酸诱发小鼠扭体反应具有镇痛作用,尽管与吲哚美辛镇痛作用相似,但地塞米松和吲哚美辛在一定程度上抑制炎症应答反应,而3-O-甲基-半乳甘露聚糖则不存在这种现象,不过由3-O-甲基-半乳甘露聚糖引起的镇痛作用机制仍然不清楚。Silveira等[13]首次报道了来源于Pleurotus sajor-caju的甲基化EPS可以减轻角叉菜所致小鼠足肿胀,表明这种EPS很可能成为一种有效的镇痛和抗炎症药剂。此外,研究发现真菌EPS具有抗细菌和抗病毒作用[52,59-60],比如香菇EPS辅助治疗抗药性的肺结核,可以提高中性粒细胞对结核菌的吞噬作用,而葡聚糖和灵芝多糖分别对风疹病毒和乙型肝炎病毒具有一定的抗性。
4.3抗氧化活性
天然抗氧化剂因在疾病预防与治疗、延缓衰老过程中发挥重要作用而广为人知,在这些天然抗氧化剂中,多糖通常被认为是抗氧化性最强的一种[61]。有研究表明真菌EPS具有抗氧化功能,可以清除自由基,比如金针菇EPS[12]、血红密孔菌EPS[62]和被毛孢属真菌EPS[14]。多糖的抗氧化活性主要取决其结构特征,包括分子质量大小、单糖组成及糖苷键连接方式等,这往往是多种因素共同作用的结果[63]。即使对于同一来源的真菌EPS,分离得到不同分子质量及单糖组成的EPS,其抗氧化性也有一定的差别。Zheng Jianqiang等[61]纯化了来源于Boletus aereus的EPS,获得3 种EPS,分别命名为Fr-I、Fr-II和Fr-III,通过体外抗氧化活性实验,发现Fr-I是其中抗氧化活性最高的EPS。
4.4益生功能
开发促进肠道有益菌群增殖,抑制有害微生物生长的益生元备受研究人员青睐,目前已经上市的各种功能性低聚糖种类繁多,包括低聚木糖、低聚半乳糖、低聚果糖等[64-65],该类益生元因进入末端结肠保留时间因素限制其应有功效[66-67]。而研究发现真菌EPS在人肠道不易被消化分解,具有防止细胞脱水、吞噬、减少血液中葡萄糖释放速率和胆固醇的积累[23,52]。另外,还有研究表明来源于酿酒酵母的可溶性和不可溶性葡聚糖具有显著降血脂的功效[52]。
开发能够应用于工业上的新型EPS,历来会引起科研人员的兴趣,随着真菌EPS研究的深入以及EPS高通量筛选平台的建立[2,68],势必会有更多新型的EPS被发现,其相应的生理学功能也将会被阐述。尽管近年来真菌EPS研究取得了丰硕的成果,但是有关真菌EPS生物合成途径和相关酶的基因调控和表达研究还相对较少,而这对于提高EPS产量研究具有很强的理论指导意义。另外EPS工业规模化生产过程中,如果菌株所利用的碳源单一,势必会增加EPS的生产成本、缺乏市场竞争力,进一步开发利用工农业废弃物,如渔业壳多糖水解物、甘蔗糖蜜等有可能提高真菌发酵EPS水平。本课题组已着手此方面研究,以期获得这方面特性的菌株以及具有特殊生物学活性的EPS。
参考文献:
[1]ZHAO W,LIU W L,LI J J,et al.Preparation of animal polysaccharides nanofibers by electrospinning and their potential biomedical applications[J].Journal of Biomedical Materials Research Part A,2015,103(2):807-818.DOI:10.1002/jbm.a.35187.
[2]RÜHMANN B,SCHMID J,SIEBER V.High throughput exopolysaccharide screening platform:from strain cultivation to monosaccharide composition and carbohydrate fingerprinting in one day[J].Carbohydrate Polymers,2015,122:212-220.DOI:10.1016/j.carbpol.2014.12.021.
[3]PATIL S P,SHIRSATH L P.Production of exopolysaccharide by an osmotolerant,thermostable and metal resistant Bacillus subtilis[J].International Journal of Current Microbiology and Applied Science,2015,4(2):965-971.
[4]FINORE I,di DONATO P,MASTASCUSA V,et al.Fermentation technologies for the optimization of marine microbial exopolysaccharide production[J].Marine Drugs,2014,12(5):3005-3024.DOI:10.3390/md12053005.
[5]RYAN P M,ROSS R P,FITZGERALD G F,et al.Sugar-coated:exopolysaccharide producing lactic acid bacteria for food and human health applications[J].Food Function,2015,6(3):679-693.DOI:10.1039/c4fo00529e.
[6]ISLAM S T,LAM J S.Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway[J].Canadian Journal of Microbiology,2014,60(11):697-716.DOI:10.1139/cjm-2014-0595.
[7]RUAS-MADIEDO P,de LOS REYES-GAVIL˘N C.Invited review:methods for the screening,isolation,and characterization of exopolysaccharides produced by lactic acid bacteria[J].Journal of Dairy Science,2005,88(3):843-856.DOI:10.3168/jds.S0022-0302(05)72750-8.
[8]MAHAPATRA S,BANERJEE D.Fungal exopolysaccharide:production,composition and applications[J].Microbiology Insights,2013,6(1):1-16.DOI:10.4137/MBI.S10957.
[9]CHEN Y L,MAO W J,TAO H W,et al.Preparation and characterization of a novel extracellular polysaccharide with antioxidant activity,from the mangrove-associated fungus Fusarium oxysporum[J].Marine Biotechnology,2015,17(2):219-228.DOI:10.1007/s10126-015-9611-6.
[10]MUKHOPADHYAY S K,CHATTERJEE S,GAURI S S,et al.Isolation and characterization of extracellular polysaccharide Thelebolan produced by a newly isolated psychrophilic antarctic fungus Thelebolus[J].Carbohydrate Polymers,2014,104:204-212.DOI:10.1016/j.carbpol.2014.01.034.
[11]ZHAO H Z,WANG J,LÜF X,et al.Chemical characterization and antitumor activity of an exopolysaccharide from Pholiota squarrosa Quel.AS 5.245[J].Food Science and Biotechnology,2015,24(2):659-664.DOI:10.1007/s10068-015-0086-z.
[12]MA Z,CUI F Y,GAO X,et al.Purification,characterization,antioxidant activity and anti-aging of exopolysaccharides by Flammulina vel utipes SF-06[J].Antonie Van Leeuwenhoek,2015,107(1):73-82.DOI:10.1007/s10482-014-0305-2.
[13]SILVEIRA M L,SMIDERLE F R,AGOSTINI F,et al.Exopolysaccharide produced by Pleurotus sajor-caju:its chemical structure an d anti-inflammatory activity[J].International Journal of Biological Macromolecules,2015,75(4):90-96.DOI:10.1016/j.ijbiomac.2015.01.023.
[14]MENG L,SUN S S,LI R,et al.Antioxidant activity of polysacchari des produced by Hirsutella sp.and relation with their chemical characteristics[J].Carbohydrate Polymers,2015,117:452-457.DOI:10.1016/j.carbpol.2014.09.076.
[15]CHEN X,SIU K C,CHEUNG Y C,et al.Structure and properties of a(1-->3)-beta-D-glucan from ultrasound-degraded exopolysaccharides of a medicinal fungus[J].Carbohydrate Polymers,2014,106:270-275.DOI:10.1016/j.carbpol.2014.02.0 40.
[16]OSINSKA-JAROSZUK M,JASZEK M,MIZERSKA DUDKA M,et al.Exopolysaccharide from Ganoderma applanatum as a promising bioactive compound with cytostatic and antibacterial properties[J].BioMed Research International,2014,2014:1-10.DOI:10.1155/2014/743812.
[ 17]MISHRA B,SUNEETHA V.Biosynthesis and hyper production of pullulan by a newly isolated strain of Aspergillus japonicus-VITSB1[J].World Journal of Microbiology and Biotechnology,2014,30(7):2045-2052.DOI:10.1007/s11274-014-1629-9.
[18]GUO S D,MAO W J,YAN M X,et al.Galactomannan with novel structure produced by the coral endophytic fungus Aspergillus ochraceus[J].Carbohydrate Polymers,2014,105:325-333.DOI:10.1016/j.c arbpol.2014.01.079.
[19]MA X K,ZHANG H,PETERSON E C,et al.Enhancing exopolysaccharide antioxidant formation and yield from Phellinus species through medium optimization studies[J].Carbohydrate Polymers,2014,107:214-220.DOI:10.1016/j.carbpol.2014.02.077.
[20]LILLO L,CAB ELLO G,CÉSPEDES C L,et al.Structural studies of the exopolysaccharide produced by a submerged culture of entomopathogenic fungus Metarhizium anisopliae[J].Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas,2014,1 3(4):359-365.
[21]SU C A,XU X Y,LIU D Y,et al.Isolation and characterization of exopolysaccharide with immunomodulatory activity from fermentation broth of Morchella conica[J].Daru Journal of Pharmaceutical Sciences,2013,21(1):1-6.DOI:10.1186/2008-2231-21-5.
[22]ZHAI X H,ZHAO A J,GENG L J,et al.Fermentation characteristics and hypoglycemic activity of an exopolysaccharide produced by submerged culture of Stropharia rugosoannulata #2[J].Annals of Microbiology,2013,63(3):1013-1020.DOI:10.1007/s13213-012-0555-z.
[23]PRATHUMPAI W,RACHATHEWEE P,KHAJEERAM S,et al.Optimization,characterization and in vitro evaluation of entomopathogenic fungal exopolysaccharides as prebiotic[J].Advances in Biochemistry,201 3,1(2):13-21.
[24]ZHU H,TIAN B Z,LIU W,et al.A three-stage culture process for improved exopolysaccharide production by Tremella fuciformis[J].Bioresource Technology,2012,116:526-528.DOI:10.1016/j.biortech.2012.03.117.
[25]WU S J,CHEN J,PAN S K.Optimization of fermentation conditions for the production of pullulan by a new strain of Aureobasidium pullulans isolated from sea mud and its characterization[J].Carbohydrate Polymers,2012,87(2):1696-1700.DOI:10.1016/j.carbpol.2011.0 9.078.
[26]MAHAPATRA S,BANERJEE D.Structural elucidation and bioactivity of a novel exopolysaccharide from endophytic Fusarium solani SD5[J].Carbohydrate Polymers,2012,90(1):683-689.DOI:10.1016/j.carbpol.2012.05.097.
[27]HE P X,GE NG L J,MAO D B,et al.Production,characterization and antioxidant activity of exopolysaccharides from submerged culture of Morchella crassipes[J].Bioprocess and Biosystems Engineering,2012,35(8):1325-1332.DOI:10.1007/s00449-012-0720-6.
[28]GAO C J,WANG Z Y,SU T T,et al.Optimisation of exopolysaccharide production by Gomphidius rutilus and its antioxidant activities in vitro[J].Carbohydrate Polymers,2012,87(3):2299-2305.DOI:10.1016/j.carbpol.2011.10.064.
[29]MARIBEL C M,HUMBERTO H S,GUSTAVO F G L,et al.Production and partial characterization of an exopolysaccharide from Ustilago maydis in submerged culture[J].African Journal of Biotechnology,2012,11(27):7079-7087.DOI:10.5897/AJB11.4047.
[30]ZHANG B B,CHEUNG P C.Use of stimulatory agents to enhance the production of bioactive exopolysaccharide from Pleurotus tuberregium by submerged fermentation[J].Journal of Agricultural and Food Chemistry,2011,59(4):1210-1216.DOI:10.1021/jf104425w.
[31]YE M,QIU T,PENG W,et al.Purification,characterization and hypoglycemic activity of extracellular polysaccharides from Lachnum calyculiforme[J].Carbohydrate Polymers,2011,86(1):285-290.DOI:10.1016/j.carbpol.2011.04.051.
[32]LIANG T W,WANG S L.Recent advances in exopolysaccharides from Paenibacillus spp.:production,isolation,structure,and bioactivities[J].Marine Drugs,2015,13(4):1847-1863.DOI:10.3390/md13041847.
[33]FENG Y L,LI W Q,WU X Q,et al.Statistical op timization of media for mycelial growth and exo-polysaccharide production by Lentinus edodes and a kinetic model study of two growth morphologies[J].Biochemical Engineering Journal,2010,49(1):104-112.DOI:10.1016/j.bej.2009.12.002.
[34]HUANG H C,LIU Y C.Enhancement of polysaccharide production by optimization of culture conditions in shake flask submerged cultivation of Grifola umbellata[J].Journal of the Chinese Institute of Chemical Engineers,2008,39(4):307-311.DOI:10.1016/j.jcice.2008.01.003.
[35]SHARMA N,PRASAD G S,CHOUDHURY A R.Utilization of corn steep liquor for biosynthesis of pullulan,an important exopolysaccharide[J].Carbohydrate Polymers,2013,93(1):95-101.DOI:10.1016/j.c arbpol.2012.06.059.
[36]SHENG L,ZHU G L,TONG Q Y.Effect of uracil on pullulan production by Aureobasidium pullulans CGMCC1234[J].Carbohydrate Polymers,2014,101(30):435-437.DOI:10.1016/j.carbpol.2013.09.063.
[37]SHENG L,ZHU G L,TONG Q Y.Mechanism study of Tween 80 enhancing the pullulan production by Aureobasidium pullulans[J].Carbohydrate Polymers,2013,97(1):121-123.DOI:10.1016/j.carbpol.2013.04.058.
[38]ZHANG B B,CHEN L,CHEUNG P C.Proteomic insights into the stim ulatory effect of Tween 80 on mycelial growth and exopolysaccharide production of an edible mushroom Pleurotus tuber-regium[J].Biotechnology Letters,2012,34(10):1863-1867.DOI:10.1007/s10529-012-0975-7.
[39]TU G W,WANG Y K,JI Y C,et al.Th e effect of Tween 80 on the polymalic acid and pullulan production by Aureobasidium pullulans CCTCC M2012223[J].World Journal of Microbiology and Biotechnology,2015,31(1):219-226.DOI:10.1007/s11274-014-1779-9.
[40]LIU J,LUO J G,YE H,et al.Production,characterization and antioxidant activities in vitro of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3[J].Carbohydrate Polymers,2009,78(2):275-281.DOI:10.1016/j.carbpol.2009.03.046.
[41]SHARMA S K,GA UTAM N,ATRI N S.Optimization,composition,and antioxidant activities of exo- and intracellular polysaccharides in submerged culture of Cordyceps gracilis(Grev.)Durieu & Mont[J].Evidence-Based Complement ary and Alternative Medicine,2015,2015:1-8.DOI:10.1155/2015/462864.
[42]SEO C,LEE H W,SURESH A,et al.Improvement of fermentative production of exopolysaccharides from Aureobasidium pullulans under various conditions[J].Korean Journal of Chemical Engineering,2014,31(8):1433-1 437.DOI:10.1007/s11814-014-0064-9.
[43]MA Z C,FU W J,LIU G L,et al.High-level pullulan production by Aureobasidium pullulans var.melanogenium P16 isolated from mangrove system[J].Applied Microbiology and Biotechnology,2014,98(11):4865-4873.DOI:10.1007/s00253-014-55 54-5.
[44]MAHAPATRA S,BANERJEE D.Optimization of a bioactive exopolysaccharide production from endophytic Fusarium solani SD5[J].Carbohydrate Polymers,2013,97(2):627-634.DOI:10.1016/j.carbpol.2013.05.039.
[45]GUO S D,MAO W J,LI Y L,et al.Structural elucidation of the exopolysaccharide produced by fungus Fusarium oxysporum Y24-2[J].Carbohydrate Research,2013,365:9-13.DOI:10.1016/j.carres.2012.09.026.
[46]POKHREL C,OHGA S.Submerged culture cond itions for mycelial yield and polysaccharides production by Lyophyllum decastes[J].Food Chemistry,2007,105(2):641-646.DOI:10.1016/j.foodchem.2007.04.033.
[47]RUPEREZ P,LEAL J A.Extracellular galactosaminogalactan from Aspergillus parasiticus[J].Transactions of the British Mycological Society,1981,77(3):621-625.DOI:10.1016/S0007-1536(81)80111-8.
[48]LIU B,SUN Z,MA X N,et al.Mutation breeding of extracellular polysaccharide-producing microalga Crypthecodinium cohnii by a novel mutagenesis with atmospheric and room temperature plasma[J].International Journal of Molecular Sciences,2015,16(4):8201-8212.DOI:10.3390/ijms16048201.
[49]KANG J X,CHEN X J,CHEN W R,et al.Enhanced production of pullulan in Aureobasidium pullulans by a new process of genome shuffling[J].Process Biochemistry,2011,46(3):792-795.DOI:10.1016/j.procbio.2010.11.004.
[50]WANG D H,CHEN F F,WEI G Y,et al.The mechanism of improved p ullulan production by nitrogen limitation in batch culture of Aureobasidium pullulans[J].Carbohydrate Polymers,2015,127(8):325-331.DOI:10.1016/j.carbpol.2015.03.079.
[51]SINGH R S,SAINI G K.Pullulan-hyperproducing color variant strain of Aureobasidium pullulans FB-1 newly isolated from phyl loplane of Ficus sp.[J].Bioresource Technology,2008,99(9):3896-3899.DOI:10.1016/j.biortech.2007.08.003.
[52]GIAVASIS I.Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals[J].Current Opinion in Biotech nology,2014,26:162-173.DOI:10.1016/j.copbio.2014.01.010.
[53]IKEKAWA T.Beneficial effects of edible and medicinal mushrooms on health care[J].International Journal of Medicinal Mushrooms,2001,3(4):1-8.DOI:10.1615/IntJMedMushr.v3.i4.20.
[54]WASSER S P.Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides[J].Applied Microbiology and Biotechnology,2002,60(3):258-274.DOI:10.1007/s00253-002-1076-7.
[55]SINGH R S,KAUR N,KENNEDY J F.Pullulan and pullulan derivatives as promising biomolecules for drug and gene targeting[J].Carbohydrate Polymers,2015,123(6):190-207.DOI:10.1016/j.carbpol.2015.01.032.
[56]SC OMPARIN A,SALMASO S,BERSANI S,et al.Novel folated and non-folated pullulan bioconjugates for anticancer drug delivery[J].European Journal of Pharmaceutical Sciences,2011,42(5):547-558.DOI:10.1016/j.ejps.2011.02.012.
[57]LI H N,BIAN S Q,HUANG Y H,et al.High drug loading pH-sensitive pullulan-DOX conjugate nanoparticles for hepatic targeting[J].Journal of Biomedical Materials Research Part A,2014,102(1):150-159.DOI:10.1002/jbm.a.34680.
[58]SMIDERLE F R,OLSEN L M,CARBONERO E R,et al.A 3-O-methylated mannogalactan from Pleurotus pulmonarius:structure and antinociceptive effect[J].Phytochemistry,2008,69(15):2731-2736.DOI:10.1016/j.phytochem.2008.08.006.
[59]EO S K,K IM Y S,LEE C K,et al.Possible mode of antiviral activity of acidic protein bound polysaccharide isolated from Ganoderma lucidum on herpes simplex viruses[J].Journal of Ethnopharmacology,2000,72(3):475-481.DOI:10.10 16/S0378-8741(00)00266-X.
[60]MASTROMARINO P,PETRUZZIELLO R,MACCHIA S R,et al.Antiviral activity of natural and semisynthetic polysaccharides on the early steps of rubella virus infection[J].Journal of Antimicrobial Chemotherapy,199 7,39(3):339-345.DOI:10.1093/jac/39.3.339.
[61]ZHENG J Q,WANG J Z,SHI C W,et al.Characterization and antioxidant activity for exopolysaccharide from submerged culture of Boletus aereus[J].Process Biochemistry,2014,49(6):1047-1 053.DOI:10.1016/j.procbio.2014.03.009.
[62]CAO J,ZHANG H J,XU C P.Culture characterization of exopolysaccharides with antioxidant activity produced by Pycnoporus sanguineus in stirred-tank and airlif t reactors[J].Journal of the Taiwan Institute of Chemical Engineers,2014,45(5):2075-2080.DOI:10.1016/j.jtice.2014.05.005.
[63]HE P X,GENG L J,WANG J Z,et al.Purification,chara cterization and bioactivity of an extracellular polysaccharide produced from Phellinus igniarius[J].Annals of Microbiology,2012,62(4):1697-1707.DOI:10.1007/s13213-012-0427-6.
[64]FALONY G,V ERSCHAEREN A,de BRUYCKER F,et al.In vitro kinetics of prebiotic inulin-type fructan fermentation by butyrate-producing colon bacteria:implementation of online gas chromatography for quantitative analysis of carbon dioxide and hydrogen gas production[J].Applied and Environmental Microbiology,2009,75(18):5884-5892.DOI:10.1128/AEM.00876-09.
[65]HERNOT D C,BOILEAU T W,BAUER L L,et al.In vitro fermentation profiles,gas production rates,and microbiota modulation as affected by certain fructans,galactooligosaccharides,and polydextrose[J].Journal of Agricultural and Food Chemistry,2009,57(4):1354-1361.DOI:10.1021/jf802484j.
[66]DOUGLAS L C,SANDERS M E.Probiotics and prebiotics in dietetics practice[J].Journal of the American Dietetic Association,2008,108(3):510-521.DOI:10.1016/j.jada.2007.12.009.
[67]SAULNIER D M,SPINLER J K,GIBSON G R,et al.Mechanisms of probiosis and prebiosis:considerations for enhanced functional foods[J].Current Opinion in Biotechnology,2009,20(2):135-141.DOI:10.1016/j.copbio.2009.01.002.
[68]SEVIOUR R J,MCNEIL B,FAZENDA M L,et al.Operating bioreactors for microbial exopolysaccharide produ ction[J].Critical Reviews in Biotechnology,2011,31(2):170-185.DOI:10.3109/07388 551.2010.505909.
YANG Tongxiang1,WU Kongyang2,CHEN Junliang1,TANG Haoguo1,KANG Huaibin1
(1.College of Food and Bioengineering,Henan University of Science and Technology,Luoyang 471023,China; 2.College of Life Science,Luoyang Normal University,Luoyang 471022,China)
Abstract:Fungal exopolysaccharide possesses high viscosity,and antioxidant,antiviral and antitumor activity,which is widely used in the food and pharmaceutical industries.In this review,we summarizes the latest progress made in China and abroad in studying exopolysaccharide-producing strains,factors influencing the production of fungal exopolysaccharide,breeding strategies for exopolysaccharide production and the function of fungal exopolysaccharide,aiming to provide some references for further investigation and application of fungal exopolysaccharide.
Key words:fungal exopolysaccharide; production; fermentation; function
中图分类号:Q939.9
文献标志码:A
文章编号:1002-6630(2016)05-0265-06
DOI:10.7506/spkx1002-6630-201605046
作者简介:杨同香(1983—),女,讲师,博士,研究方向为乳品科学与工程。E-mail:txyamy@163.com
基金项目:河南省高等学校重点科研基金项目(15A180050);河南科技大学博士科研启动基金项目(13480043);河南科技大学青年科学基金项目(2015QN038)
收稿日期:2015-06-12