ZHU Lijuan1, GE Pingzhen1,2, WEI Cheng1, LI Chenglong1,3, LIU Shuzhen1,4, ZHOU Caiqiong1,5,*
(1. College of Food Science, Southwest University, Chongqing 400715, China; 2. Bijie Institute of Agricultural Sciences, Bijie 551700, China; 3. Chongqing Brewery Co. Ltd., Chongqing 400000, China; 4. Fujian Anjoy Food Limited by Share Ltd., Xiamen 361028, China; 5. Chongqing Engineering Research Center of Regional Food, Chongqing 400715, China)
Abstract:Fermented rice-chili, a fermented product produced by lactic acid bacteria in southwest China, is produced by anaerobic fermentation of a mixture of crushed fresh red chili, rice flour, salt and other ingredients for a certain time. Chili, rich in polyphenols, may release bound polyphenols during fermentation. Thus, antioxidant activity of fermented rice-chili in cells and its effect on lipid peroxidation in the liver of mice were studied. Results showed total reducing power and oxygen radical absorbance capacity (ORAC) of fermented rice-chili reached their maximum value at 15 and 60 d, respectively. 1,1-diphenyl-2-picryl-hydrazyl (DPPH) free radical scavenging activity of fermented rice-chili at 15 d was higher but not significantly than at 60 d (P > 0.05). However, fermented rice-chili at 15 d showed significantly lower (P < 0.05) inhibitory effect on lipid peroxidation in the liver than at 60 d. Fermented rice-chili at 60 d had significantly lower (P < 0.05) carbonyl content than the control + AAPH group, and its percentage inhibition against bovine serum albumin (BSA) oxidation was 2.38 times as high as that at 15 d. For hemolysis delay time, the sample at 15 d declined faster than that at 60 d with the difference being not significant, and the longest hemolysis delay time was achieved at 0.5 mg/mL. Polyphenol content and percentage inhibition against BSA oxidation were positively correlated (P < 0.01). The antioxidant capacity of fermented rice-chili in cells could be increased by increasing fermentation time, but polyphenol content was not enough to estimate the cellular antioxidant capacity. These data suggest that chemical antioxidant tests in vitro alone are not enough to evaluate antioxidant capacity, and comprehensive evaluation of food antioxidant properties using a cell model can give more objective judgment.
Key words:fermented rice-chili; antioxidant effect; percentage inhibition against oxidation; erythrocyte hemolysis; liver lipid peroxidation
Fermented rice-chili, a traditional fermented chili product in southwest China, is produced by the anaerobic fermentation of a mixture of crushed fresh red chili, rice flour, salt and other ingredients. Chili contains many polyphenols, carotenoids, capsaicinoids, VC and VE with antioxidant activity[1-2]. Fermented rice-chili is a natural malolactic fermented product produced under anaerobic condition, which promotes an increase in organic acids and releases bound polyphenol. These two reactions changed the antioxidant activity. Research has shown that bound phenolic compounds, including flavonoids, correlated well with antioxidant activity[3]. Therefore, studying changes in antioxidant activity of fermented rice-chili during different fermentation periods is meaningful for its functionalization development and industrial production.
Currently, numerous studies on antioxidant prosperities of chili and cereal reported that antioxidant activity was quantified by antioxidant assays in vitro[4-8], such as 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay, the total oxyradical scavenging assay, the total reducing power assay, iron reducing power (FRAP) assay, the oxygen radical absorbance capacity (ORAC) assay[9-11]and peroxide value (POV)[12]. The nutritional quality, flavor formation and security of fermented rice-chili were investigated in our previous studies[13-15], the results demonstrated that fermented ricechili released bound polyphenol and produced large amounts of organic acids during fermentation, the antioxidant effect on fermented rice-chili in vitro was relatively high after 30 d of fermentation. However, these antioxidant assays in vitro are based on the structural characteristic of antioxidants, they reflect the antioxidant activity indirectly through some characteristic product produced by bond breaking of hydrogen ions or metal chelating ion[16], and chemical assays do not necessarily reflect the cellular physiological conditions and do not consider the bioavailability and metabolism issues, thus antioxidant assays in vitro are not all-inclusive. Currently, some studies on antioxidant capacity using cellular antioxidant evaluation system have been reported already[17-19]. Due to high polyunsaturated fatty acid (PUFA) content in erythrocyte membranes and using hemoglobin molecules with redox activity to transport oxygen, and it was easily attacked by free radical[20]. These results verify that introducing the cellular antioxidant evaluation system to assess the characteristics of fermented rice-chili, which is helpful to understand the changes on antioxidant activity of local traditional fermented chili during fermentation and will promote the industrial development of fermented chili. Currently less relevant research has been reported yet.
Here, cellular antioxidant activity (CAA) assays was employed to investigate antioxidant activity of fermented rice-chili according to the method of Wolfe[21-22]and Song Wei[23]et al., and red blood cells were applied as experimental cells to study the protective effect on protein oxidative damage and the inhibition effect onthe liver lipid peroxidation of fermented rice-chili when antioxidant ability in vitro was stronger[24]. This study may provide important information for monitoring changes in antioxidant activity and choosing proper fermentation time during the fermentation of rice-chili.
1.1 Animals, materials and reagents
SD rats were clean grade, weighed 180–200 g, purchased from Chongqing Tengxin Bill Experimental Animals Sales Co. Ltd., experimental animals license number: SCXK (Chongqing) 2007-0008.
Chili (Capsicum annuum L. erjingtiao), and taro werepurchased at Tiansheng farmer’s market in Beibei District of Chongqing. Iodized salt and rice were purchased at Yonghui Supermarket in Beibei District of Chongqing.
DPPH free radical TCI Chemical Industry (Shanghai); Trolox ((±)-6-hydroxy-2,5,7,8-tetramethyl -chromane-2-carboxylic acid) Sigma-Aldrich (US); 2,2-azobis(2-methylpropionamidine) dihydrochloride (AAPH) Adamas Reagent Co. Ltd. (Switzerland); ethanol, guanidine hydrochloride, 2,4-dinitrophenylhydrazine and ethyl acetate (AR) Chengdu Kelong Chemical Co. Ltd.; bovine serum albumin (BSA) and other biochemical reagents Shanghai Bao Biotechnology Co. Ltd..
1.2 Equipments and instruments
Synergy H1 microplate reader Gene Co. Ltd. (US); UV-2450 UV-Vis spectrophotometer Shimadzu (Japan); DHG-9140A DHG series heating and drying oven Shanghai Qixin Scientific Instrument Co. (China); 722 spectrophotometer Shanghai Jing-Hua Technology Instrument Co. Ltd. (China); RE-52A rotary evaporator Shanghai Ya Rong Biochemical Instrument Factory (China); TDL80-2B centrifugal machine Shanghai Anting Acientific Anstrument Factory (China).
1.3 Methods
1.3.1 Fermentation of rice-chili
Chilies were washed, drained and crushed. Rice was smashed by pulverizer and then sieved through 40 mesh screen. The two materials were mixed at 1:1 mass ratio of chilies to rice powder. Salt was added at 5% based on the mass of the two materials. After mixing all materials, the mixture was loaded to a tank. The sealed tank was then inverted and water-sealed. The fermentation was carried out at 20-30 ℃ controlled by air condition. Samples were taken at different fermentation periods.
1.3.2 Antioxidant activity of fermented rice-chili at different fermentation periods in vitro
1.3.2.1 Preparation of sample solution
The sample solution was preparedby the method of Wang Xichun[25]. Ten gram fermented rice-chili at different fermentation periods was extracted by 80 mL 50% ethanol, at 50 ℃ for 2 h. The mixture was filtrated after cooling, and filtrate was centrifuged at 3 000 r/min for 30 min, then volume to 100 mL with the ethanol content of 50%, and the solution was stored at 4 ℃ for further experiments.
1.3.2.2 Measurement of total reducing power
According to the Prussian blue method of Zhang Qingfeng et al.[26], 1 mL of each sample solution was added to a centrifuge tube, and then 2.5 mL phosphate buffer (0.2 mol/L, pH 6.6) and 2.5 mL 1 g/100 mL iron potassium cyanide solution were added to each tube. The tubes were mixed vigorously, and incubated in a water bath at 50 ℃ for 20 min. Then the tubes were taken out, and 2.5 mL 10 g/100 mL trichloroacetic acid (TCA) was added to each tube to terminate the reaction. The mixture was centrifuged at 3 000 r/min for 10 min, then 2.5 mL supernatant was transferred to a test tube. Then, 0.5 mL 3 g/100 mL FeCl3solution and 2.5 mL H2O were added in order. After mixing, standing for 10 min, absorbance value of the solution was measured at 700 nm. 1 mL extraction solvent was taken as blank control. Results are reported as the mean of 3 replicates. 1 mL Trolox solution ranging from 100 to 500 μmol/L was used to make standard curve according to the previous step. The extracted phosphate buffer solution was taken instead of Trolox solution as blank control obtaining the regression equation of standard curve, y = 0.000 9x-0.001 2 (R2= 0.990 9). The total reducing power of each sample was calculated by equation (1).
Where ciis the concentration of the extract/(mol/L); V is the volume of the extract/mL; m is the mass of the sample/g.
1.3.2.3 Measurement of ORAC
ORAC of every sample was measured by the method of Kim et al.[27]. Concentrations of Trolox standard solution used as a control standard were 10, 20, 30, 40 and 50 μmol/L, respectively, they were obtained by the dilution of 0.5 mmol/L Trolox solution. The sample solution was diluted to a suitable concentration. 25 μL sample solution, blank (pH 7.4, 75 mmol/L phosphate buffer solution (PBS) or appropriate extraction solvent) or standard Trolox solution, and 150 μL 81.63 nmol/L sodium fluorescein were added into 96 orifice-plate successively, the plate was incubated in 37 ℃preheated microplate reader for 20 min. The plate was then removed out and added quickly with 25 μL of 153 mmol/L AAPH solution, and carried to fluorescence microplate reader for scanning. Excitation at 485 nm and emission at 525 nm, and readings were recorded every minute during 60 min. The reaction time was horizontal coordinates while the fluorescence intensity was the vertical coordinates to make the fluorescence decay curve. The net area under the curve (AUC) is calculated by equation (2).
Where f0is the initial fluorescence intensity when t = 0 min; fiis the fluorescence intensity when t=i min (the reaction can be considered over when the fluorescence intensity ≤15 000).
The ORAC was calculated according to equation (3).
Where cTroloxwas molarity of Trolox solution/(mol/L); csamplewas molarity of sample/(mol/L).
1.3.2.4 Relative DPPH free radical scavenging capacity
Relative DPPH free radical scavenging capacity (RDSC) values were determined by a high-throughput method of Cheng Zhihong et al.[28]. The concentrations of Trolox solution were 0.00, 6.25, 12.50, 25.00, 37.50 and 50.00 μmol/L, respectively, obtaining from the dilution of 0.5 mmol/L Trolox solution. Fermented rice-chili extract was diluted to a suitable concentration with phosphate buffer solution (75 mmol/L, pH 7.4 PBS). 100 μL solvent, Trolox standard solution of different concentrations, and suitable concentration solution of fermented rice-chili extract were added to 96 orifice-plate, respectively. Then 100 μL of 0.208 mmol/L DPPH free radical solution was added to multifunction microplate reader quickly. After shaking, the absorbance was measured at 515 nm within 40 min, each sample repeated three times. The DPPH free radical scavenging capacity was calculated by equation (4).
Where Asample, Acontroland Ablankrepresent the absorbance of different concentrations extract, Trolox and solvent at 515 nm when the reaction time is t min (t = 0-40 min).
The DPPH free radical scavenging rate at different reaction time was obtained according to the reaction time and equation (4). The figure of DPPH free radical scavenging rate was made and the AUC was calculated according to equation (5).
Where f0is the DPPH free radical scavenging rate when t = 0 min; fiis the DPPH free radical scavenging rate when t = 40 min.
According to different concentrations of Trolox and corresponding AUC, the Trolox standard curve of the sample RDSC was established to obtain the linear equation: y = 34.080x + 245.42 (R2= 0.996 0).
RDSC of each sample within 40 min was calculated by equation (6).
Where cTroloxwas molarity of Trolox solution/(mol/L); ρsamplewas concentration of sample/(g/L). RDSC of each extract was represented as Trolox equivalent of micromoles of per gram extract (μmol TE/g fermented rice-chili extract).
EC50is the concentration of extracted solution in the reaction system when the DPPH free radical scavenging rate was 50%. EC50can be obtained through the figure of different concentrations extract and corresponding DPPH free radical scavenging rate at 40 min.
1.3.3 Antioxidant activity of fermented rice-chili in the cells
1.3.3.1 Preparation of sample solution
The supernatant, extracted by the method 1.3.2.1, was freeze-dried. Yield: the sample at 15 d was (21.18 ± 0.72) g/100 g md(A) and the sample at 60 d was (18.61 ± 0.62) g/100 g md(B).
1.3.3.2 The protective effect of protein oxidative damage
The procedure was carried out according to Dorman et al.[29]. BSA was made into 1.25 mg/mL solution with 100 mmol/L KH2PO4-KOH buffer solution (pH 7.4). The reaction system included 2.4 mL of 1.25 mg/mL BSA solution, 0.3 mL of 2.0 mg/mL extracted solution, 0.3 mL of 100 mmol/L AAPH solution. The solution was mixed and incubated at 37 ℃for 90 min. The inhibition degree of protein oxidation was measured by the method 2,4-dinitrophenylhydrazine (DNPH). 0.5 mL of 20 mmol/L DNPH (dissolved in 2.0 mol/L HCl solution) was added to the centrifuge tube loaded with 1 mL reaction mixture, placed in dark for 1 h at room temperature (shaking every 10 min), 0.5 mL of 20 g/100 mL TCA solution was added to terminate the reaction, keeping the tube on ice for 10 min. The mixture was then centrifuged at 3 000 × g for 10 min. The resultant precipitation was washed three times with 3.0 mL of ethanol and ethyl acetate (V/V was 1:1) solution to remove residual 2,4-dinitrophenylhydrazine. The protein precipitation was redissolved with 1 mL of 6 mol/L guanidine hydrochloride (pH 2.3), incubating at 37 ℃ for 10 min. The absorbance of solution was then measured at 370 nm. The carbonyl content was calculated by equation (7).
Where c represented the carbonyl content/(mol/L); A370nmwas the absorbance of the solution; L was optical length/m; ε is the coefficient 22 000 L/(mol·cm).
The buffer solution was used as a control of protein oxidation instead of AAPH (control), the solution was used as a control instead of the extract solution (control + AAPH),and the buffer solution was used as a blank instead of the bovine serum albumin solution (blank), the protein oxidation inhibition rate was calculated by equation (8).
Where Asampleand Acontrol+AAPHwere absorbance of tested sample and control measured at 370 nm, respectively.
1.3.4 Inhibition effect on the liver lipid peroxidation in mice
1.3.4.1 Preparation of rat liver homogenate
SD rats were fasted overnight and put to death the following day. Blood was collected for further analysis and liver was removed quickly and rinsed repeatedly with 4 ℃ pre-cooling saline to wipe off the blood. The fat and connective tissue were removed, and the liver was dried with filter paper, and weighed. The liver was homogenized on ice to make into tissue homogenate of 10% (m/m) with saline. The tissue homogenate was centrifuged at 3 000 r/min for 10 min at 4 ℃. The resulting supernatant was prepared for subsequent experiments.
1.3.4.2 Inhibition effect on the liver lipid peroxidation
Inhibition effect of fermented rice-chili extract on liver lipid peroxidation was investigated according to the method of Tai et al.[30], but with some modification. Briefly, 0.1 mL liver homogenate and 1 mL extracted solution of different concentrations (0.5, 1.0, 2.0, 5.0, 10.0 mg/mL) were added into a test tube incubating at 37 ℃ for 2 h. 1 mL TCA solution (20 g/100 mL) was added to terminate the reaction. The mixture was vortexed and centrifuged at 5 000 r/min for 10 min. 1 mL supernatant was transferred to a tube containing 1 mL of TBA solution (0.67 g/100 mL). The tube was incubated in boiling water for 15 min and subsequently cooled to room temperature in ice water, and the absorbance of the solution was measured at 532 nm. Malondialdehyde (MDA) as the final product of lipid peroxidation reaction, it will produce red products reacting with TBA, and it can be quantified by colorimetric method. So the content of MDA can be used as an indicator of lipid peroxidation. The inhibition rate of MDA was calculated by equation (9).
Where Asample, Acontrol+AAPHand Ablankwere absorbances of tested smaple, control and blank measured at 532 nm, respectively.
1.3.5 Protective effect on cells oxidative damage
1.3.5.1 Preparation of cell suspension
Blood of SD rats collected from section 1.3.4.1 was centrifuged at 2 500 r/min for 25 min at 4 ℃ to make red blood cells separate from plasma. The red blood cells were rinsed twice with pre-cooling phosphate buffer (pH 7.4) by centrifuging at 2 500 r/min for 10 min to remove the plasma, platelets and buffy coat. The red blood cells was diluted with phosphate buffer (pH 7.4) at 1:100 for making cell suspension. Being counted by hemocytometer, the number of red blood cells obtained in cell suspension was 1.52×1011cells/L.
1.3.5.2 Cell hemolysis
Cell hemolysis was investigated according to the method of Takebayashi et al.[31], but with some modification. Briefly, 50 μL cell suspension (1 g/100 mL) and 100 μL fermented rice-chili extracted solution with different concentrations or 100 μL PBS buffer were added to 96 orifice-plate. 100 μL H2O and 50 μL cell suspension were added as a complete hemolysis control. After shaking culture at 37 ℃ for 10 min, 50 μL AAPH (160 mmol/L, dissolved in phospate buffer, fresh made) was added to the plate, and the absorbance (At) was measure at 660 nm every 10 min within 3 h. The result of red blood cell complete rate was calculated by equation (10), hemolysis delay time (ΔT) was calculated by equation (11).
Where Atand A0represented the optical density value at t and 0 min of hemolysis, respectively; A0,CHrepresented the absorbance at 0 min of completely hemolysis .
Where HT50, obtaining from the hemolysis curve, is the time required when hemolysis is 50%/min.
1.3.6 Determination of polyphenol content
Total polyphenols was evaluated by Folin-Ciocalteu assay[32]and represented with gallic acid equivalents contained in per 100 g fermented rice-chili (mg gallic acid equivalents/100 g md), abbreviated as mg GAE/100 g.
1.4 Data analysis
Each sample was measured three times and the results were expressed with ± s, analyzed by the least significant difference in SPSS 20.0.
± s, analyzed by the least significant difference in SPSS 20.0.
2.1 Total reducing power and ORAC of fermented ricechili at different fermented period
Table 1 Total reducing power and ORAC during rice-chili fermentation
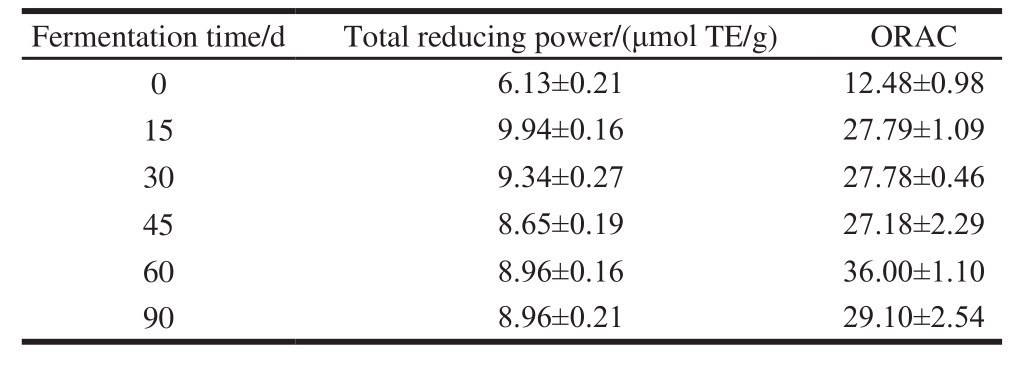
The total reducing power and ORAC of fermented ricechili were both relatively high at 15–90 d (Table 1). The total reducing power decreased slowly after reaching the peak at 15 d, and the total reducing power of the sample fermented 15-90 d was 162.20%–141.11% of the sample that not fermented; ORAC increased rapidly after fermented 15 d, and reaching the peak at 60 d, and ORAC of the sample fermented 15–90 d was 217.79%–288.46% of the sample that not fermented, both the peak appeared at 15 and 60 d, respectively. These suggest that fermentation may produce some materials or metabolites possessing stronger antioxidant activity. However, with the extension of time, these materials may turn into other substances or the activity reduced.
2.2 RDSC of fermented rice-chili
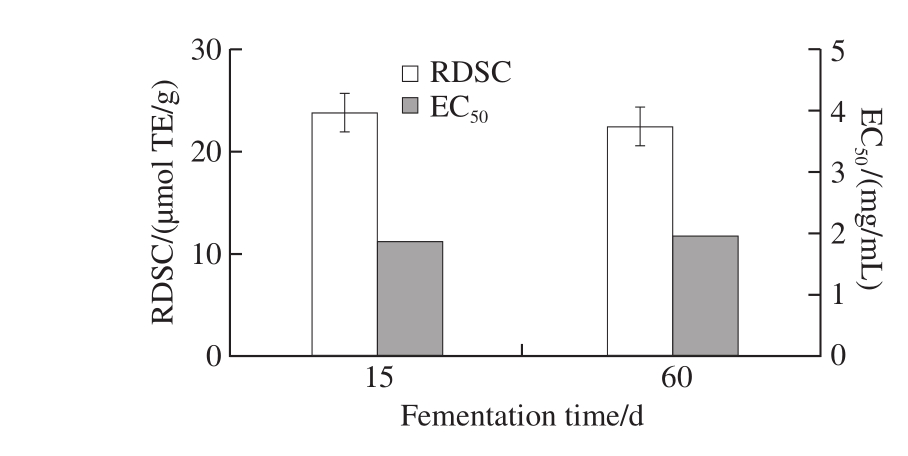
Fig. 1 RDSC and EC50of fermented rice-chili extract
DPPH free radical scavenging test was widely used for choosing biological active ingredients, and it was an important indicator to evaluate the free radical scavenging capacity of antioxidants. Results of further analysis on RDSC of fermented rice-chili at 15 and 60 d were shown in Fig. 1. RDSC of fermented rice-chili after 15 and 60 d were 23.72 and 22.45 μmol TE/g, respectively. The scavenging ability on DPPH free radical of fermented rice-chili at 15 d was higher than that at 60 d, but not significantly (P > 0.05). This may be related to microbial phase and population[33-34]. EC50of fermented rice-chili were 1.88 and 1.95 mg/mL at 15 and 60 d, respectively. As shown in Fig. 1, there was no significant difference between the sample at 15 and 60 d in chemistry antioxidant effect. Thus, fermented rice-chili at 15 and 60 d were chosen for subsequent studies.
2.3 Protective effect on protein oxidative damage of fermented rice-chili
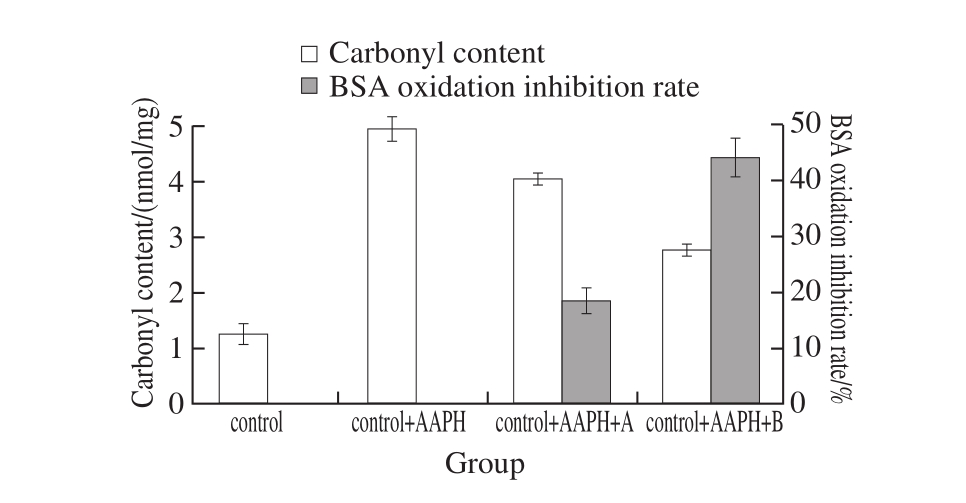
Fig. 2 Protective effect of fermented rice-chili extracts on protein oxidative damage
Fig. 2 shows the effect on the carbonyl formation during protein oxidation of fermented rice-chili extract. After adding AAPH, carbonyl content increased, and it was 3.9 times of control, indicating the degree of protein oxidation was enhanced. When added extract A and B, carbonyl levels were lower than control + AAPH group and higher than control group, of which sample at 60 d and added with extract B was significantly lower than control + AAPH group (P < 0.05), showing higher protein anti-oxidation of control + AAPH + B. After analyzing the protective effect on protein oxidative damage, when concentration of all the extract was 2 mg/mL, BSA oxidation inhibition rate of the sample at 60 d was 2.4 times of that at 15 d. These results are consistent with the notion that wine pomace and sulfite inhibit protein oxidation in beef patties during high oxygen atmosphere storage[35].
2.4 Inhibition effect on liver lipid peroxidation of fermented rice-chili
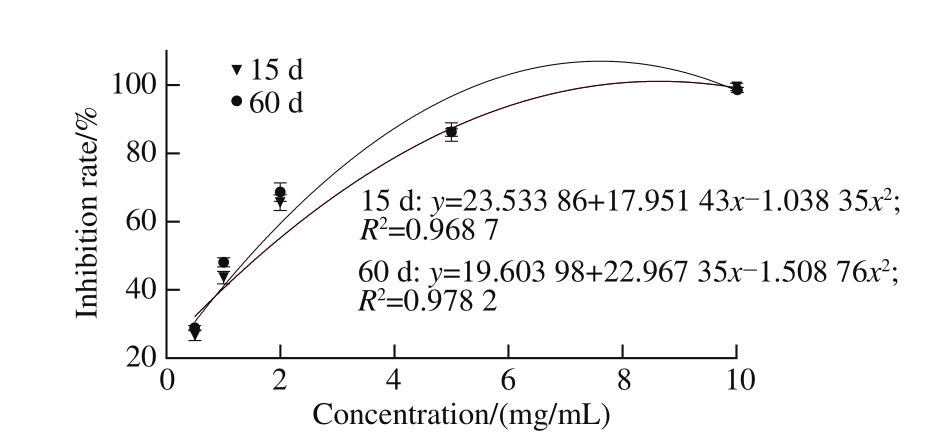
Fig. 3 Inhibitory effect of fermented rice-chili extracts on lipid peroxidation in liver
As shown in Fig. 3, with the concentration of each extract increased, the MDA content was lower, and the inhibition effect on the liver lipid peroxidation was enhanced. Through analysis on the half maximal inhibitory concentration (IC50) of the inhibition effect with the lipid peroxidation, IC50of the sample at 15 and 60 d were (1.57 ± 0.05)and (1.34 ± 0.04) mg/mL, respectively. The inhibition effect with the lipid peroxidation of the sample at 60 d was significantly stronger than at 15 d (P < 0.05). These results are similar to García-Lomillo et al.[36]who reported that wine byproducts showed high global antioxidant activities (ABTS assay), and successfully delayed the onset of lipid oxidation in the Rancimat test.
2.5 The effect on hemolysis curve of fermented rice-chili extract
Our studies serve as a proof-of-concept that the time of fermented rice-chili extract maintained the cell integrity was related to the concentration and fermentation time. When the concentration of the extract was below 1.0 mg/mL, the time maintained the cell intact was prolonged with increasing fermented rice-chili extract concentration. When the concentration of the extract was above 1.0 mg/mL, the intact cells of the sample at 15 d decreased gradually with increasing extract concentration and fermentation time. When the concentration of the extract was more than 2.0 mg/mL, the intact cells of the sample at 60 d decreased gradually with an increase in extract concentration and fermentation time (Fig. 4).
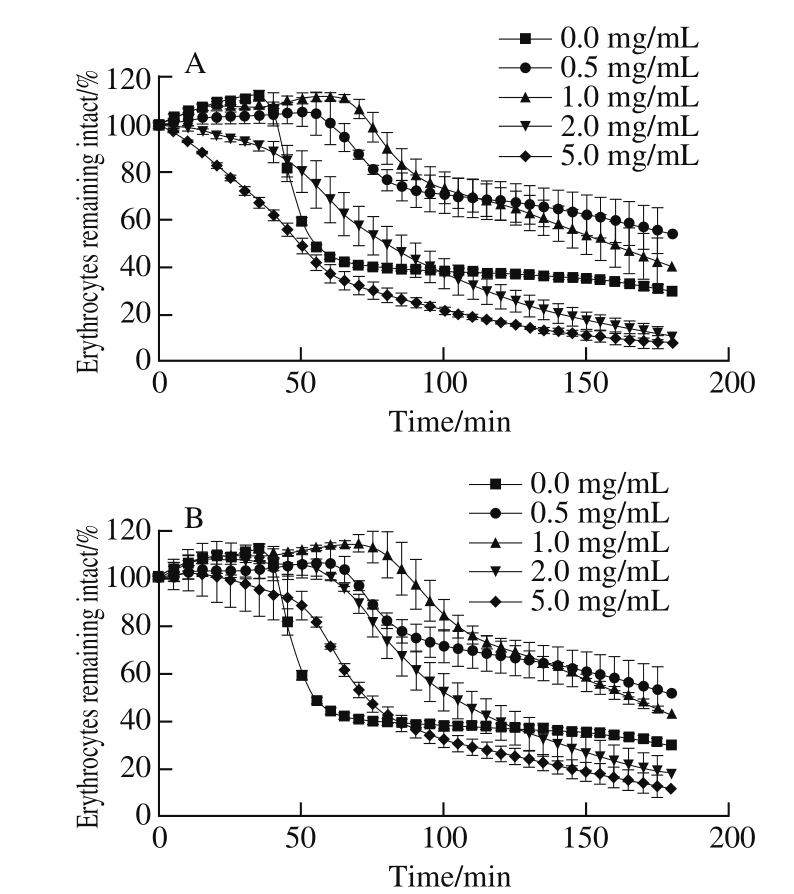
Fig. 4 Hemolysis curves with fermented rice-chili extracts at 15 (A) and 60 d (B)
Table 2 Hemolysis delay time (ΔT) of fermented rice-chili extracts min
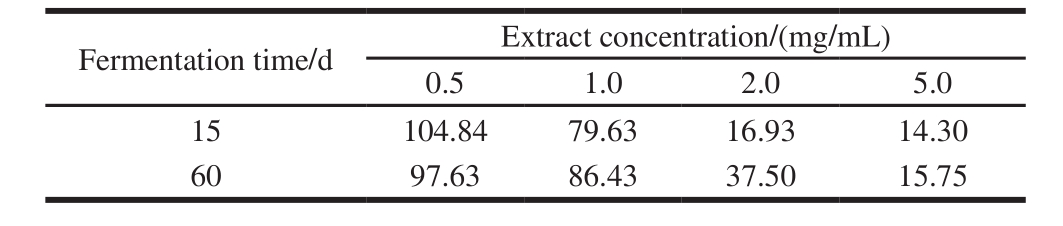
Delay time of hemolysis (ΔT) was longer, indicating that the inhibition effect of extract on hemolysis was stronger at the concentration. Analysis on hemolysis delay time (ΔT) of fermented rice-chili extract at different extract concentrations was shown in Table 2. ΔT of the sample at 15 and 60 d were declined rapidly with increasing concentration. ΔT was longest when the concentration was 0.5 mg/mL, were 104.84 and 97.63 min of the sample at 15 and 60 d, but the difference was not significant (P > 0.05). With the concentration increasing, the sample at 15 d declined faster than at 60 d. From the influence on cell integrity of the extract and ΔT, it is known that the effect on hemolysis curve of fermented ricechili extract was affected by fermentation time and extract concentration.
2.6 Correlation analysis of polyphenol and antioxidant capacity of fermented rice-chili
Numerous previous studies show that polyphenol, flavonoids and other phytochemicals possess strong scavenging ability in various free radical[29]. After analyzing on polyphenol content of extract, the samples at 15 and 60 d had (10.39 ± 0.64) and (11.93 ± 0.29) mg GAE/g, respectively. Through its antioxidant correlation analysis, the results were shown in Table 3. The change in polyphenol content had a positive correlation with RDSC (P > 0.05), and a negative correlation with the inhibition effect on the liver lipid peroxidation (P > 0.05), and a significantly positive correlation with the BSA oxidation inhibition rate (P < 0.01), which showed that the polyphenol content can’t be used to evaluate the antioxidant effect in the cells of the samples.
Table 3 Correlation(r ) analysis between polyphenols content and antioxidant capacity of fermented rice-chili
Note:**. Correlated extremely significantly (P < 0.01).
The total reducing power and ORAC of fermented rice-chili reached a high level after 15 d of fermentation and reached their maximum values at 15 and 60 d, respectively. Further analysis on RDSC showed that relative DPPH radical scavenging activity of fermented rice-chili at 15 d was higher but not significantly (P > 0.05) than at 60 d. this showed that the effect on antioxidant of fermented rice-chili in vitro had no difference between the sample at 15 and 60 d. These results are similar to the report of Dorman et al.[29]that water-soluble food-related botanical extract are of potential pro-oxidant activities using both DNA and BSA as substrates.
We have noticed that fermented rice-chili extract inhibite the protein oxidation in cellular antioxidant, and theinhibiting effect of the sample at 60 d was significantly lower than control+AAPH group (P < 0.05), oxidation inhibition rate to BSA of fermented rice-chili extractat 60 d was 2.4 times of 15 d, indicating higher protein anti-oxidation. Next, the inhibition effect on the liver lipid peroxidation was explored, and the results suggest that MDA value was lower with the extract concentration increased, the inhibition effect on the liver lipid peroxidation of the sample at 60 d was significantly stronger than 15 d (P < 0.05). Last, the effect of the red cell hemolysis was examined, and the result indicated the time for maintaining cells integrity was related to the concentration of fermented rice-chili extract, the time was the longest when the concentration was 1.0 mg/mL. Overall, the difference was not significant (P > 0.05) between the samples at 15 and 60 d, and this showed that antioxidant effect in the cells difference when the antioxidant effect of fermented rice-chili in vitro was strong. These results suggest fermented rice-chili at 60 d has a stronger antioxidant capacity in the cells. VC and VE, important antioxidant nutrients, were measured during fermentation. VC content declined with the fermentation processing, and it drops rapidly at the early stage of the fermentation (0-30 d), then tend to be flat. While VE content showed a rise first followed by a decline, it reached the maximum values at 30 d. Changes of VC and VE content influenced the antioxidant activity of fermented rice-chili a lot. Through correlation analysis on the main antioxidant ingredient polyphenol content and antioxidant effect of the extract, we found that the polyphenol content couldn’t be used to evaluate the antioxidant effect of the samples.
Overall, our studies revealed the complexity of antioxidant effect and the limitations of antioxidant assays in vitro. All of these are connected with the mechanism of antioxidant in the cells and in vitro. Antioxidant assays in vitro are based on the structural characteristics of antioxidants, they reflect the antioxidant activity indirectly through some characteristic product produced by bond breaking of hydrogen ions or metal chelating ion[16], and chemical assays do not necessarily reflect the cellular physiological conditions and do not consider the bioavailability and metabolism issues[37], and antioxidants are easily affected in vivo environment (such as temperature, pH value, microorganisms, etc.), as well, substances of high antioxidant ability in vitro may not be able to enter the cell to scavenge free radicals, it would tend to low antioxidant ability in the cells. This result is similar to the report of Dordević et al.[38]that the DPPH free radical and Fe3+scavenging capacity of buckwheat was high while the inhibition effect on the liver lipid peroxidation was low. What’s more, the mechanism of antioxidant action is not only by scavenging free radicals, but also by inhibiting free radicals produce or by raising the level of endogenous antioxidants to achieve antioxidant effect, thus antioxidant experiments in vitro are not comprehensive[39]. Food antioxidant capacity is determined by different antioxidant components and is a result of joint effect[40], additional and synergistic effect would occur among different components[41]. Different antioxidant components intercoordinated and scavenged radical jointly to avoid the damage from cell oxidative damage[42]. As a result, single antioxidant component couldn’t be used to evaluate food antioxidant capacity.
Because of the complexity of the antioxidant researches and we can’t use antioxidant assays in vitro to evaluate the antioxidant activity of food or functional component alone. In recent years, a new method based on cell biological assay was established for evaluation of antioxidant activity, and it developed extensively and was applied widely. Based on chemical oxidation, this study established the cell model to evaluate the food antioxidant capacity. Our results suggest that whether single antioxidant component or antioxidant assays in vitro can’t be used to evaluate food antioxidant capacity. This is consistent with the notion of Wolfe[21], Dorman[29], Liu Ruihai[43]et al.. Therefore, the evaluation of food active ingredients and antioxidant effect need more researches to establish a more scientific and objective system.
[1] CHEN Long. Study on antioxidant and antimicrobial activity of ten kinds of pepper in Yunnan[D]. Kunming: Kunming University of Science and Technology, 2010: 1-4.
[2] MATERSKA M, PERUCKA I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.)[J]. Journal of Agricultural and Food Chemistry, 2005, 53(5): 1750-1756. DOI:10.1021/jf035331k.
[3] LEE Y, HOWARD L R, VILLALÓN B. Flavonoids and antioxidant activity of fresh pepper (Capsicum annuum) cultivars[J]. Journal of Food Science, 2006, 60(3): 473-476. DOI:10.1111/j.1365-2621.1995. tb09806.x.
[4] CAO G H, ALESSIO H M, CUTLER R G. Oxygen-radical absorbance capacity assay for antioxidants[J]. Free Radical Biology and Medicine, 1993, 14(3): 303-311. DOI:10.1016/0891-5849(93)90027-R.
[5] REGOLI F, NIGRO M, BOMPADRE S, et al. Total oxidant scavenging capacity (TOSC) of microsomal and cytosolic fractions from Antarctic, Arctic and Mediterranean scallops: differentiation between three potent oxidants[J]. Aquatic Toxicology, 2000, 50(1/2): 13-25. DOI:10.1016/S0166-445X(99)00070-3.
[6] ZHAO Yanhong, LI Jianke, LI Guoxiu. Activity evaluation methods in vitro of natural antioxidants and optimizations[J]. Food Science, 2008, 29(6): 64-69. DOI:10.3321/j.issn:1002-6630.2008.06.008.
[7] BENZIE I F, SZETO Y T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay[J]. Journal of Agricultural and Food Chemistry, 1999, 47(2): 633-636. DOI:10.1021/jf9807768.
[8] PRIOR R L, WU X, SCHAICH K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements[J]. Journal of Agricultural and Food Chemistry, 2005, 53(10): 4290-4302. DOI:10.1021/jf0502698.
[9] LUO X J, PENG J, LI Y J. Recent advances in the study on capsaicinoids and capsinoids[J]. European Journal of Pharmacology, 2011, 650(1): 1-7. DOI:10.1016/j.ejphar.2010.09.074.
[10] PERUCKA I, MATERSKA M. Phenylalanine ammonia-lyase and antioxidant activities of lipophilic fraction of fresh pepper fruits Capsicum annum L.[J]. Innovative Food Science and Emerging Technologies, 2001, 2(3): 189-192. DOI:10.1016/S1466-8564(01)00022-4.
[11] ARSLAN D, ÖZCAN M M. Dehydration of red bell-pepper (Capsicum annuum L.): change in drying behavior, colour and antioxidant content[J]. Food and Bioproducts Processing, 2011, 89(4): 504-513. DOI:10.1016/j.fbp.2010.09.009.
[12] QU J S, WAN Q J, Antioxidation activity of capsicum from different region in Guizhou province[J]. Journal of the Chinese Cereals and Oils Association, 2009, 24(2): 113-115.
[13] SHAO Wei, ZHOU Yuan, LI Meihua, et al. Application of HACCP in fermented cayenne production[J]. China Brewing, 2000, 19(6): 32-34.
[14] ZHANG Yaxiong, HU Bin, SHAO Wei. Study on constituents changes in fermented cayenne[J]. Food Science, 2000, 21(12): 74-76. DOI:10.3321/j.issn:1002-6630.2000.12.025.
[15] WANG W. Study on production technology of fermented chilli pure fermentation[D]. Chongqing: Southwestern University, 2013: 6-8.
[16] BARTASIUTE A, WESTERINK B H C, VERPOORTE E, et al. Improving the in vivo predictability of an on-line HPLC stable free radical decoloration assay for antioxidant activity in methanol-buffer medium[J]. Free Radical Biology and Medicine, 2007, 42(3): 413-423. DOI:10.1016/j.freeradbiomed.2006.11.010.
[17] YOKOMIZO A, MORIWAKI M. Effects of uptake of flavonoids on oxidative stress induced by hydrogen peroxide in human intestinal Caco-2 cells[J]. Bioscience, Biotechnology, and Biochemistry, 2006, 70(6): 1317-1324. DOI:10.1271/bbb.50604.
[18] SEREM J C, BESTER M J. Physicochemical properties, antioxidant activity and cellular protective effects of honeys from southern Africa[J]. Food Chemistry, 2012, 133(4): 1544-1550. DOI:10.1016/ j.foodchem.2012.02.047.
[19] ANGELINO D, GENNARI L, BLASA, et al. Chemical and cellular antioxidant activity of phytochemicals purified from olive mill waste waters[J]. Journal of Agricultural and Food Chemistry, 2011, 59(5): 2011-2018. DOI:10.1021/jf103881b.
[20] SADRZADEH S M, GRAF E, ANTER S S, et al. Hemoglobin, a biologic fenton reagent[J]. Journal of Biological Chemistry, 1984, 259(23): 14354-14356.
[21] WOLFE K L, LIU R H. Structure- activity relationships of flavonoids in the cellular antioxidant activity assay[J]. Journal of Agricultural and Food Chemistry, 2008, 56(18): 8404-8411. DOI:10.1021/jf8013074.
[22] WOLFE K L, KANG X, HE X, et al. Cellular antioxidant activity of common fruits[J]. Journal of Agricultural and Food Chemistry, 2008, 56(18): 8418-8426. DOI:10.1021/jf801381y.
[23] SONG Wei, DERITO C M, LIU M K S, et al. Cellular antioxidant activity of common vegetables[J]. Journal of Agricultural and Food Chemistry, 2010, 58(11): 6621-6629. DOI:10.1021/jf801381y.
[24] BLASA M, ANGELINO D, GENNARI L, et al. The cellular antioxidant activity in red blood cells (CAA-RBC): a new approach to bioavailability and synergy of phytochemicals and botanical extracts[J]. Food Chemistry, 2011, 125: 685-691. DOI:10.1016/ j.foodchem.2010.09.065.
[25] WANG Xichun. Study on solid fermentation with high fibrinolytic activity and its antioxidant properties[D]. Wuxi: Jiangnan University, 2007: 21.
[26] ZHANG Qingfeng, ZHANG Zhongrong, CHEUNG H Y. Antioxidant activity of Rhizoma smilacis Glabrae extracts and its key constituentastilbin[J]. Food Chemistry, 2009, 115(1): 297-303. DOI:10.1016/ j.foodchem.2008.11.053.
[27] KIM D B, SHIN G H, LEE Y J, et al. Assessment and comparison of the antioxidant activities and nitrite scavenging activity of commonly consumed beverages in Korea[J]. Food Chemistry, 2014, 151: 58-64. DOI:10.1016/j.foodchem.2013.11.034.
[28] CHENG Zhihong, MOORE J, YU Liangli. High-throughput relative DPPH radical scavenging capacity assay[J]. Journal of Agricultural and Food Chemistry, 2006, 54(20): 7429-7436. DOI:10.1021/jf0611668.
[29] DORMAN H J D, HILTUNEN R. Antioxidant and pro-oxidant in vitro evaluation of water-soluble food-related botanical extracts[J]. Food Chemistry, 2011, 129(4): 1612-1618. DOI:10.1016/ j.foodchem.2011.06.017.
[30] TAI Z, CAI L, DAI L, et al. Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia[J]. Food Chemistry, 2011, 126(4): 1648-1654. DOI:10.1016/j.foodchem.2010.12.048.
[31] TAKEBAYASHI J, IWAHASHI N, ISHIMI Y, et al. Development of a simple 96-well plate method for evaluation of antioxidant activity based on the oxidative haemolysis inhibition assay (OxHLIA)[J]. Food Chemistry, 2012, 134(1): 606-610. DOI:10.1016/j.foodchem.2012.02.086.
[32] DEWANTO V, WU X, ADOM K K, et al. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity[J]. Journal of Agricultural and Food Chemistry, 2002, 50(10): 3010-3014. DOI:10.1021/jf0115589.
[33] KARILUOTO S, AITTAMAA M, KORHOLA M, et al. Effects of yeasts and bacteria on the levels of folates in rye sourdoughs[J]. International Journal of Food Microbiology, 2006, 106: 137-143. DOI:10.1016/j.ijfoodmicro.2005.06.013.
[34] MOORE J, CHENG Z, HAO J, et al. Effects of solid-state yeast treatment on the antioxidant properties and protein and fiber compositions of common hard wheat bran[J]. Journal of Agricultural and Food Chemistry, 2007, 55(25): 10173-10182. DOI:10.1021/jf071590o.
[35] WOLFE K L, LIU R H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements[J]. Journal of Agricultural and Food Chemistry, 2007, 55(22): 8896-8907. DOI:10.1021/jf0715166.
[36] GARCÍA-LOMILLO J, SKIBSTED L H, JONGBERG S. Effect of skin wine pomace and sulfite on protein oxidation in beef patties during high oxygen atmosphere storage[J]. Food and Bioprocess Technology, 2016, 9(3): 1-11. DOI:10.1007/s11947-015-1649-y.
[37] LIU Ruihai, FINLEY J. Potential cell culture models for antioxidant research[J]. Journal of Agricultural and Food Chemistry, 2005, 53(10): 4311-4314.
[38] DORDEVIĆ T M, ŠILER-MARINKOVIĆ S S. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals[J]. Food Chemistry, 2010, 119(3): 957-963. DOI:10.1016/j.foodchem.2009.07.049.
[39] XIA C Y, GUO X H, LI F H, et al. The advance of cellular antioxidant activity assay in the evaluation of food antioxidant activity[J]. Food Science, 2012, 33(15): 297-302.
[40] PÉREZ-JIMÉNEZ J, ARRANZ S, TABERNERO M, et al. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: extraction, measurement and expression of results[J]. Food Research International, 2008, 41(3): 274-285. DOI:10.1016/ j.foodres.2007.12.004.
[41] LIU Ruihai. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals[J]. The American Journal of Clinical Nutrition, 2003, 78(3): 517-520. DOI:10.3969/ J.ISSN.1003-5788.2013.05.003.
[42] LI H, ZHONG H Y, FANG X Z, et al. Changes of the antioxidants in the tea seed during ripening process[J]. Food and Machinery, 2013(5): 6-9.
[43] LIU Ruihai, FINLEY J. Potential cell culture models for antioxidant research[J]. Journal of Agricultural and Food Chemistry, 2005, 53(10): 4311-4314. DOI:10.1021/jf058070i.
粳米鲊海椒的抗氧化活性及其对小鼠肝脏脂质过氧化作用的影响
朱丽娟1,葛平珍1,2,韦 诚1,李成龙1,3,刘淑贞1,4,周才琼1,5,*
(1.西南大学食品科学学院,重庆 400715;2.毕节市农业科学研究所,贵州 毕节 551700;3.重庆啤酒股份有限公司,重庆 400000;4.福建安井食品股份有限公司,福建 厦门 361028;5.重庆市特色食品工程技术研究中心,重庆 400715)
摘 要:粳米鲊海椒是以新鲜红辣椒经过破碎后与米粉等配料以一定比例混合并添加适量盐后厌氧发酵一定时间而成的一种中国西南地区地方特色的自然乳酸发酵辣椒制品。由于辣椒富含多酚,以及发酵过程中结合型多酚可能会释放等,因此研究了鲊海椒化学抗氧化活性及对肝脏脂质过氧化作用的影响。对不同发酵时段鲊海椒研究结果显示,总还原力和氧自由基吸收能力(oxygen radical absorbance capacity,ORAC)在发酵15 d后即达到较高水平,其峰值分别出现在发酵15 d和发酵60 d。进一步抗氧化活性分析显示,发酵15 d样品清除1,1-二苯基-2-三硝基苯肼自由基的能力强于发酵60 d样品,但无显著差异(P>0.05)。细胞抗氧化作用研究显示,发酵60 d样品羰基含量显著低于对照+偶氮二异丁脒盐酸盐组(P<0.05),对牛血清白蛋白氧化抑制率是发酵15 d样品的2.38 倍;红细胞溶血延迟时间随处理质量浓度增加快速下降,发酵15 d样品下降速率快于发酵60 d样品,质量浓度为0.5 mg/mL时溶血延迟时间相对最长,发酵15 d和60 d样品差异不显著(P>0.5);发酵60 d样品对肝脏脂质过氧化的抑制作用显著强于发酵15 d样品(P<0.05),表明延长发酵时间有利于提升细胞抗氧化能力。相关性分析结果显示,不能用多酚含量判断样品的细胞抗氧化作用。本研究表明不能单独用化学抗氧化方法评价抗氧化能力,通过建立细胞模型综合评价食物的抗氧化性能更客观判断食物的抗氧化能力。
关键词:粳米鲊海椒;抗氧化作用;氧化抑制率;红细胞溶血;肝脏脂质过氧化
References:
收稿日期:2016-12-19
基金项目:重庆市特色食品工程技术研究中心能力提升项目(cstc2014pt-gc8001)
作者简介:朱丽娟(1992—),女,硕士研究生,研究方向为食品化学与营养学。E-mail:694076266@qq.com
中图分类号:TS201.2
文献标志码:A
文章编号:1002-6630(2017)13-0229-09
引文格式:
*通信作者:周才琼(1964—),女,教授,博士,研究方向为食品化学与营养学。E-mail:zhoucaiqiong@swu.edu.cn
DOI:10.7506/spkx1002-6630-201713038
ZHU Lijuan, GE Pingzhen, WEI Cheng, et al. Antioxidant activity of fermented rice-chili in cells and its effect on liver lipid peroxidation in mice[J]. 食品科学, 2017, 38(13): 229-237.
DOI:10.7506/spkx1002-6630-201713038. http://www.spkx.net.cn
ZHU Lijuan, GE Pingzhen, WEI Cheng, et al. Antioxidant activity of fermented rice-chili in cells and its effect on liver lipid peroxidation in mice[J]. Food Science, 2017, 38(13): 229-237. (in English with Chinese abstract) DOI:10.7506/spkx1002-6630-201713038. http://www.spkx.net.cn