YI Ruokun1,2, FENG Xia1,2, LI Guijie1,2, PENG Deguang3, MU Jianfei1,2, FU Chenggang1,2, ZHAO Xin1,2,*
(1. Chongqing Collaborative Innovation Center for Functional Food, Chongqing Engineering Research Center of Functional Food, Chongqing Engineering Laboratory for Research and Development of Functional Food, Chongqing University of Education, Chongqing 400067, China; 2. College of Biological and Chemical Engineering, Chongqing University of Education, Chongqing 400067, China; 3. Chongqing Enterprise Engineering Research Center of Ba-lotus Breeding and Deep Processing, Chongqing 400041, China)
Abstract:The aim of this study was to investigate the effect of alkaloids from Ba lotus seeds (Nelumbo nucifera Gaertn) on HCl/ethanol-induced gastric mucosal injury and to elucidate the underlying mechanism. Mice were intragastrically administered with alkaloids from Ba lotus seed (ABLS) and gastric mucosal injury was induced in mice by HCl/ethanol. Serum cytokine and other index levels were examined by using commercial kits, and the gastric tissue was determined by reverse transcription polymerase chain reaction (RT-PCR) and western blot assay. The gastric mucosal injury area, percentage inhibition, gastric secretion volume and pH of gastric juice in the 100 mg/(kg·d) ABLS treated-mice were 1.13 mm2, 84.5%, 0.52 mL and 3.3, respectively. Additionally, serum levels of interleukin (IL)-6, IL-12, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), motilin (MOT), substance P (SP) and endothelin-1 (ET-1) in these mice were lower than those in the control mice, while the levels of somatostatin (SS) and vasoactive intestinal peptide (VIP) were higher than those in the control mice. The treated mice had higher superoxide dismutase (SOD) activity, and lower MDA level in gastric tissues than the control ones. ABLS also could increase the mRNA and protein expression levels of inhibitor kappaB-α (IκB-α), epidermal growth factor (EGF), epidermal growth factor receptor (EGFR), neuronal nitric oxide synthase (nNOS), endothelial NOS (eNOS), Mn-SOD, Gu/Zn-SOD and catalase (CAT), and decrease nuclear factor kappaB (NF-κB) and inducible NOS (iNOS) expression in gastric tissues compared to those in the control group. This study has demonstrated that ABLS treatment attenuated gastric mucosal injury due to their antioxidant capacity.
Key words:Ba lotus (Nelumbo nucifera Gaertn); anti-inf l ammatory; alkaloids; antioxidant
Ba lotus (Nelumbo nucifera Gaertn) is mainly originated in Chongqing, China. Its seed can be used as functional food or traditional Chinese medicine because it has certain effects of reducing blood pressure, alleviating insomnia and stopping vomiting. It also has a variety of functional compounds including carbohydrates and proteins, a number of vitamins, minerals, fatty acids and alkaloid compounds. Its abundant alkaloid compounds have many functional effects and hence are of great utilization value[1-2].
Ethanol is highly corrosive for gastric mucosa. It can enhance the permeability of cellular membrane, destroy the mucous layer and mucous cells on the surface, and hence has the ability to ruin the physiological environment required for normal metabolism of gastric mucosa, and as a result, cause ulceration[3]. Not only ethanol itself but also its metabolites can cause the injury. Ethanol can immerse neutrophils in gastric mucosa and release myeloperoxidase, oxidative free radicals, active oxidative metabolites such as O2−· and protease. It also adheres to vascular endothelium and causes mucosal injury through great vessel occlusion[4]. A variety of enzyme systems, including many isozymes of alcohol dehydrogenase, cytochrome P450 and catalase (CAT), are involved in the metabolism of ethanol in gastrointestinal tract. Ethanol is metabolized to acetaldehyde in the gastric mucosa, the involvement of acetaldehyde and gastric mucosal proteins leads to the damage of gastric mucosa[5]. After being catalyzed by ADH (antidiuretic hormone), ethanol generates acetaldehyde and the later generates oxygen free radicals under the action of xanthenes oxidation and hence causes gastric mucosal injury. Free radical reaction is closely related to the occurrence and development of ulcer. In addition, ethanol is destructive to gastric mucosa, and weakens the protective effect of the defensive factors of gastric mucosa. This is related to the ethanol-induced oxidative stress response of gastric tissue[6].
Alkaloid compounds extensively exist in plants. Their contents are particularly high in certain parts. Alkaloid can serve as an active oxygen quencher: it gains energy by colliding with O2and inactivates1O2and turns it into3O2[7]. Alkaloid compounds in multiple plants have high antioxidant activity in vitro in scavenging ·OH, O2−· and organic 1,1-diphenyl-2-picryhydrazyl (DPPH) radical[8]. Certain alkaloid compounds resist oxidation through inhibiting lipid peroxidation and mechanisms like oxidase system. It has been indicated that liensinine, a major extract from lotus seed, has favorable scavenging activity to oxygen free radical[9]. The resulting antioxidation may alleviate the organism injury caused by oxidative stress.
Ba lotus seed is used in food processing and production of healthy foods. Its alkaloid may have the same effect of alkaloids present in seeds of other nymphaeaceae plants. In this study, the inhibitory effect of alkaloid from Ba lotus seed(ABLS) on mice with ethanol-induced gastric mucosal injury was examined. The biochemical indexes in mouse blood and tissues were tested in order to observe the antioxidation, inhibiting effect on inflammation and influence of ABLS on the defensive factors of gastric mucosa, determine its preventive effect and mechanism of action. The research result and analysis will provide further development of functional effect of Ba lotus seed with theoretical foundation.
1.1 Animals, materials and reagents
Fifty male ICR mice (7-week old) were purchased from the Experimental Animal Center of Chongqing Medical University (certif i cate number: SYXK(Yu)2012-0001).
Ba lotus seed was purchased in local market (Chongqing, China).
Interleukin-6 (IL-6), IL-12, tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) serum cytokine kits BioLegend, Inc. (USA); motilin (MOT), somatostatin (SS), substance P (SP), vasoactive intestinal peptide (VIP) serum kit Beijing Puer Weiye Biotechnology Co. Ltd. (Beijing, China); endothelin- 1(ET-1) serum kit, superoxide dismutase (SOD), nitric oxide (NO), malondialdehyde (MDA) tissue kit Nanjing Jiancheng Bioengineering Institute (Jiangsu, China); RNeasy kit Qiagen (Germany); Trizol reagent Invitrogen (USA); avian myeloblastosis virus reverse transcriptase GE Healthcare (England); protein assay kit Bio-Rad (USA); nuclear factor kappaB (NF-κB), inhibitor kappaB-α (IκB-α), epidermal growth factor (EGF), epidermal growth factor receptor (EGFR), euronal nitric oxide synthase (nNOS), endothelial nitric oxide synthase (eNOS), iNOS, Mn-SOD, Gu/Zn-SOD, CAT antibody Abcam (USA); enhanced chemiluminescence Amersham Life Sciences (USA).
1.2 Instruments and equipments
D550 digital camera Canon (Japan); SevenEasy pH meter Mettler-Toledo (Switzerland); iMark microplate reader, T100 thermal cycler Bio-Rad (USA).
1.3 Methods
1.3.1 Extraction of ABLS
One thousand gram of Ba lotus seed had been extracted by 80% ethanol ref l ux (10 L) twice, each for 1 h. Then the solution was fi ltered and the fi ltrate obtained at the fi rst time and at the second time had been combined and concentrated to 1 L under reduced pressure. The extracting solution was passed through the 732 kation-exchange column. After full adsorption, the water soluble impurities were eliminated with distilled water and then eluted with 80% ethanol, and the eluent was collected. After concentration and desiccation, the eluent was eluted with 80% ethanol containing 2% of ammonium hydroxide. The total alkaloid of lotus germ was obtained after second concentration and desiccation.
1.3.2 Determination of ABLS content
As a reference 5 mg of neferine was precisely weighted and put into 5 mL bottle, 0.1 mol/L hydrochloric acid was added to dissolve and dilute till the scale, then solution was shaken up. 0.20, 0.25, 0.10, 0.30, 0.35, 0.40 and 0.50 mL of the control solution was drawn in 10 mL bottle, and 0.1 mol/L hydrochloric acid was added to dilute to the scale, light absorption value at 276 nm wavelength was measured. With 0.1 mol/L hydrochloric acid solution as a blank reference ratio, absorbance (A) as the ordinate and neferine concentration (c) as the abscissa, with which, the standard curve was drawn, which has the regression equation: A = 0.016 47c + 0.014 57 (r2= 0.999 9). Precision extraction of ABLS, to 0.1 mol/L hydrochloric acid dissolution, dissolved in 25 mL bottle diluted to the scale, and mixed well. The absorbance value at 276 nm wavelength was measured, and the total alkaloid content was calculated according to the regression equation.
1.3.3 Animal experiments
Mice were divided into 5 groups with 10 mice in each group. In the first 30 days, the mice in normal and control groups were administered with only distilled water (0.1 mL/(10 g·d), based on body weight, the same below) by intragastric administration; the ABLS groups were treated with two concentrations (50 and 100 mg/(kg·d)) of ABLS and the mice in drug control group (ranitidine) were treated with 50 mg/(kg·d) ranitidine by intragastric administration. At the 30thday post treatment, all mice were fasted for 12 h. The mice of ABLS and drug control groups were treated by intragastric administration of HCl/ethanol (60% in 150 mmol/L HCl, 10 mL/kg) gastric mucosal injury inducement reagent, then all the mice were sacrificed after 1 h under deep ether anesthesia. The gastric mucosal injury area (mm2) of stomach was observed under a digital camera and the images were analyzed by ImageJ software. Gastric juice pH was measured using a pH meter following a 10-times dilution. The mouse blood was centrifugalized (4 000 r/min, 10 min), then the serum was collected.

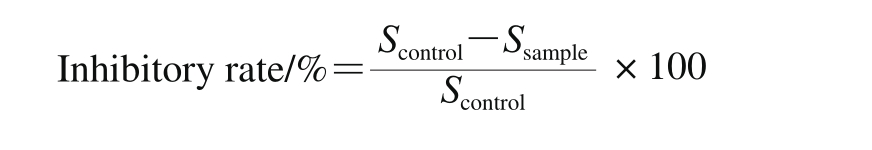
Where Scontrolis gastric mucosal injury area of control group mice; Ssampleis gastric mucosal injury area of sample group mice.
These experiments followed a protocol approved by the Animal Ethics Committee of Chongqing Medical University.
1.3.4 Analysis of serum cytokines using ELISA
The levels of cytokine, including IL-6, IL-12, TNF-α and IFN-γ, in serum were determined by enzymelinked immunosorbent assay (ELISA) according to the manufacturer’s instructions. The biotinylated antibody reagent and the mouse serum samples were added to 96-well plates and incubated at 37 ℃ in CO2for 2 h. Following washing with phosphate buffer saline (PBS), streptavidinperoxidase solution was added and the plate then placed for 30 min at room temperature. The absorbance at 450 nm was measured using a microplate reader.
1.3.5 Analysis of MOT, SS, SP and VIP levels in serum
The content of MOT, SS, SP and VIP in serum samples were measured using the biochemistry kits following the manufacturer’s instructions. ET-1 serum level was also determined by ELISA kit.
1.3.6 Gastric tissues antioxidation levels analysis
Gastric tissues antioxidation SOD, NO and MDA levels were determined by the experiment kits.
1.3.7 Reverse transcription-polymerase chain reaction (RT-PCR) assay
Total gastric tissue RNA was isolated using Trizol reagent according to the manufacturer’s recommendations. The RNA was digested with RNase-free DNase for 15 min at 37 ℃and purified using an RNeasy kit according to the manufacturer’s protocol. cDNA was synthesized from 2 μg of total RNA by incubation at 37 ℃ for l h with avian myeloblastosis virus reverse transcriptase with random hexanucleotides according to the manufacturer’s instruction. Sequences of primers used to specif i cally amplify the targeted genes were as follows: 5’-CAC TTA TGG ACA ACT ATG AGG TCT CTG G-3’ (forward) and 5’-CTG TCT TGT GGA CAA CGC AGT GGA ATT TTA GG-3’ (reverse) for NF-κB; 5’-GCT GAA GAA GGA GCG GCT ACT-3’(forward) and 5’-TCG TAC TCC TCG TCT TTC ATG GA-3’(reverse) for IκB-α; 5’-GCC AAG CTC AGA AGG CTA C-3’(forward) and 5’-CAG GCC AGC CTC GTC TCA T-3’(reverse) for EGF; 5’-TCG GTG CTG TGC GAT TTA-3’(forward) and 5’-TTT CTG GCA GTT GCT CCT C-3’ (reverse) for EGFR; 5’-GAA TAC CAG CCT GAT CCA TGG AA-3’ (forward) and 5’-TCC TCC AGG AGG GTG TCC ACC GCA TG-3’ (reverse) for nNOS; 5’-GGA GAG GCT GCA TGA CAT TG-3’ (forward) and 5’-GGT AGA GCC ATA GTG GAA TGA C-3’ (reverse) for eNOS; 5’-AGA GAG ATC GGG TTC ACA-3’ (forward) and 5’-CAC AGA ACT GAG GGT ACA-3’ (reverse) for iNOS; 5’-TTC AAT AAG GAG CAG GGA C-3’ (forward) and 5’-CAG TGT AAG GCT GAC GGT TT-3’ (reverse) for Mn-SOD; 5’-GAA GAG AGG CAT GTT GGA GA-3’ (forward) and 5’-CCA ATT ACA CCA CGA GCC AA-3’ (reverse) for Cu/Zn-SOD; 5’-AGA TAC TCC AAG GCG AAG GTG-3’(forward) and 5’-AAA GCC ACG AGG GTC ACG AAC-3’(reverse) for CAT. GAPDH was amplified as an internal control gene with the following primers: 5’-CGG AGT CAA CGG ATT TGG TC-3’ (forward) and 5’-AGC CTT CTC CAT GGT CGT GA-3’ (reverse). The PCR amplification was performed in a thermal cycler. The PCR products were separated in 1.0% agarose gels and visualized with ethidium bromide staining[10].
1.3.8 Western blot analysis
Total gastric protein was obtained using RIPA buffer, the extracted protein concentration was determined by the protein assay kit. Aliquots of the lysate containing 30-50 µg of protein were separated by 12% sodium dodecyl sulfonate (SDS) and then electrotransferred onto a nitrocellulose membrane. Antibodies against NF-κB, IκB-α, EGF, EGFR, nNOS, eNOS, iNOS, Mn-SOD, Gu/Zn-SOD and CAT were incubated for 1 h, followed by incubation with corresponding the horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Blots were washed three times with PBS-T and then developed with enhanced chemiluminescence[11].
1.4 Statistical analysis
The SAS v9.1 statistical software package was used for the analysis. The data presented as the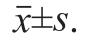 The differences between the mean values for individual groups were assessed by a one-way ANOVA with Duncan’s multiple range tests. P < 0.05 was considered to a statistically signi fi cant difference.
The differences between the mean values for individual groups were assessed by a one-way ANOVA with Duncan’s multiple range tests. P < 0.05 was considered to a statistically signi fi cant difference.
2.1 ABLS content
Neferine (C38H44N2O6) is as the standard determination of the content of alkaloids in crude ABLS. Results showedthat the purity of the extraction of crude ABLS reaches 67.4%. It plays an important role in further biological experiments and physiologically active ingredient is ABLS.
2.2 Stomach appearance
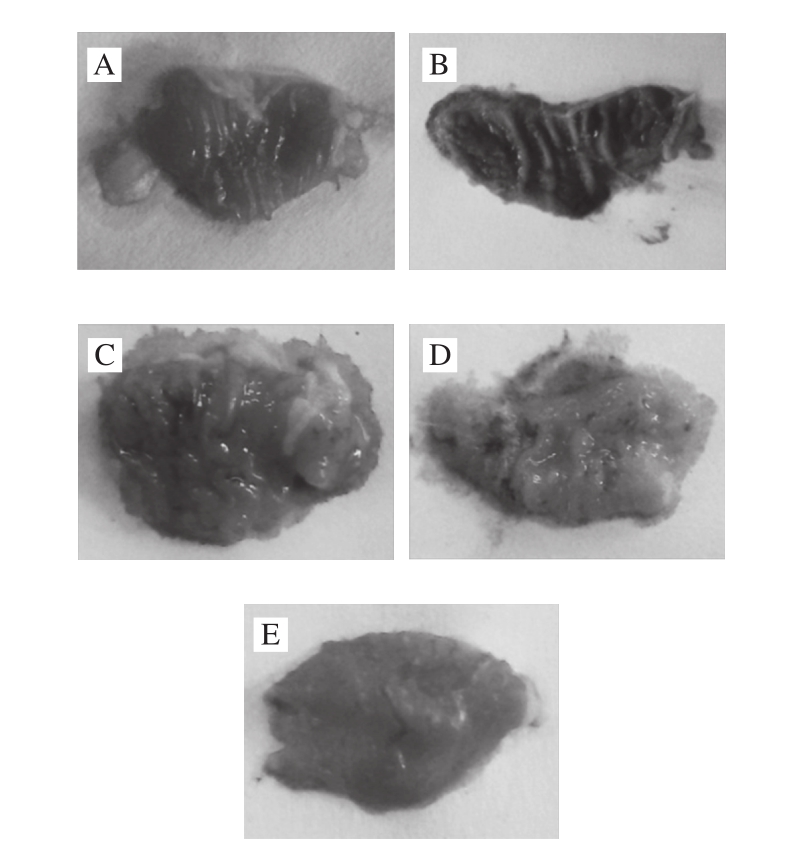
Fig. 1 Photographs of stomach of mice with HCl/ethanol-induced gastric mucosal injury treated with ABLS
A-E. Normal, control, 50mg/(kg·d) ABLS, 100 mg/(kg·d) ABLS and drug control group.
Table 1 Effect of ABLS administration on HCl/ethanol-induced gastric mucosal injury in mice
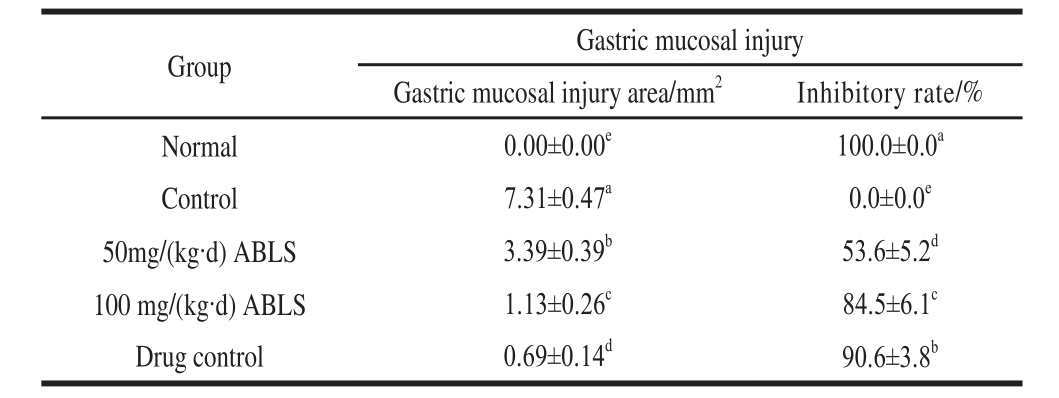
Note: Different letters in the same column indicate significant difference (P<0.05). The same below.
Ethanol-induced gastric mucosal injury can be determined by observing the damaged area of gastric wall[11]. In this study, ABLS reduced the damaged area of gastric mucosa and alleviated the influence of ethanol. The control, ABLS and ranitidinetreated mice were injured by HCl/ethanol. The gastric mucosal injured area of control group mice was highest (Table 1, Fig. 1); ABLS could reduce the injury area, and the 100 mg/(kg·d) ABLS treated mice showed a lower injury area close to that of drug (50 mg/(kg·d) ranitidine) treated mice. The 100 mg/(kg·d) ABLS and 50 mg/(kg·d) ranitidine treated mice also showed higher inhibitory rates (84.5 and 90.6%) on gastric mucosal injury.
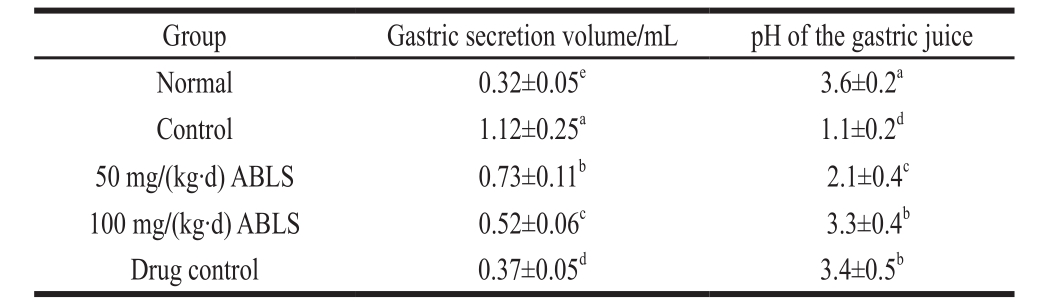
2.3 Gastric secretion volume and determination of pH of gastric juice
Ethanol stimulates gastric acid secretion after it enters the stomach and the gastric acid increases the injury. Therefore, increase of gastric acid and decline in pH value are significant evidence of gastric mucosal injury[12]. As shown in Table 2, mice in normal group showed the highest pH of the gastric juice and lowest gastric secretion volume. After HCl/ethanol induced gastric mucosal injury, the pH of the gastric juice was decreased and gastric secretion volume was increased. ABLS could reduce these changes, the pH of the gastric juice of mice in ABLS group were signif i cantly higher than that of the control group (P < 0.05), and the gastric secretion volumes were signif i cantly lower than that of mice in control group (P < 0.05).
Table 2 Effect of ABLS administration on gastric secretion volume and pH of gastric juice in mice with HCl /ethanol-induced gastric mucosal injury
2.4 Cytokine levels of IL-6, IL-12, TNF-α and IFN-γ in serum
Table 3 Effect of ABLS administration on serum levels of cytokine IL-6, IL-12, TNF-αand IFN-γin mice with HCl/ethanol-induced gastric mucosal injury pg/mL
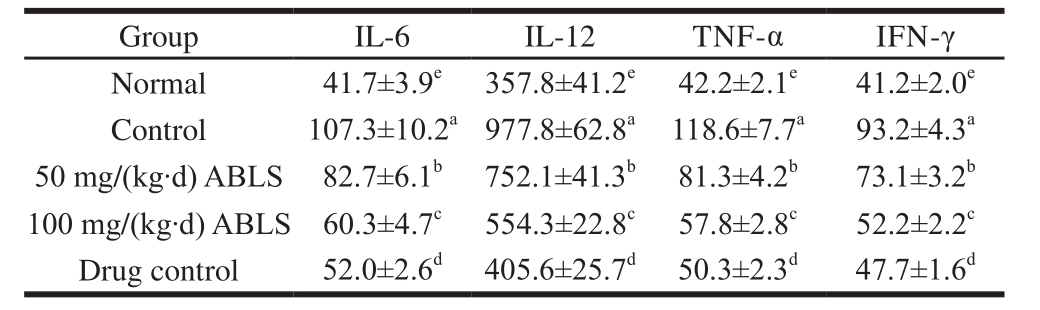
After being induced gastric mucosal injury, mice in the control group showed the highest levels of cytokine IL-6, IL-12, TNF-α and IFN-γ in serum (Table 3). ABLS treatment significantly (P < 0.05) decreased the levels of these cytokines, and high concentration of 100 mg/(kg·d) ABLS showed the lower levels of IL-6, IL-12, TNF-α and IFN-γ compared to those of 50 mg/(kg·d) ABLS treatment. The mice in 100 mg/(kg·d) ABLS group showed the similar reducing activities on IL-6, IL-12, TNF-α and IFN-γ to those of mice in drug control group. IL-6, IL-12, TNF-α and IFN-γ are all cytokines related to inflammation. Ethanol-induced gastric mucosal injury resulted in inf l ammatory response and the inflammation aggravated with the increase of injury[13].After the tissues were injured, the endothelial cells secrete a large quantity of IL-6 and IL-12 and hence directly trigger the inflammatory response. It also aggravated the inflammation by stimulating release of other inflammatory mediators and give out more IL-6 and IL-12[14]. TNF-α is a cytokine with a variety of bioactivities. Its substantial production and release in vivo will ruin the immunological balance of organism and cause gastric mucosal injury together with other inf l ammatory factors[15]. The IFN-γ is a primary cause for peptic ulcer and changes the protective effect of gastric mucosal injury. Abundant IFN-γ can aggravate gastric ulcer and reduce protective effect of organism on gastric mucosa[10]. It is also shown that, ethanol-induced gastric mucosal injury enhances the level of IL-6, IL-12, TNF-α and IFN-γ[11].
2.5 Serum levels of MOT, SS, SP, VIP and ET-1
Table 4 Effect of ABLS administration on serum levels of MOT, SS, SP, VIP and ET-1 in mice with HCl/ethanol-induced gastric mucosal injury
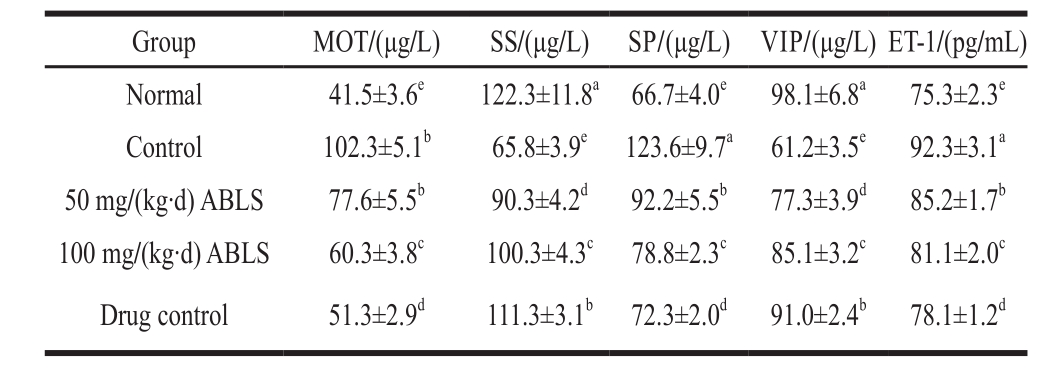
The mice in control group exhibited the higher levels of MOT, SP, ET-1 in serum compared to those in mice of other groups (Table 4), and the 100 mg/(kg·d) ABLS group mice showed lower levels of MOT, SP and ET-1 than those of mice in control and 50 mg/(kg·d) ABLS groups. For the SS and VIP, their levels were lowest in mice of control group, and ABLS signif i cantly (P < 0.05) raised their levels. MOT and SP are stimulant gastrointestinal hormones whose levels are increased once they are stimulated. When stimulated by ethanol, MOT and SP cause large gastric acid secretion, hence leading to higher acidity and more severe gastric mucosal injury[13]. SS and VIP are inhibitive gastrointestinal hormones which restrain gastric acid secretion. After the tissues were impaired, the levels of SS and VIP were declined and their inhibitory effects on gastric acid were also weaken[16]. ET, a major factor which regulates cardiovascular function, plays an important role in maintaining basal vascular tone and steady state of cardiovascular system. ET-1 is mainly expressed in endothelial cells. ET-1 and NO are two major vascoactive mediators produced by vascular endothelial cells. They interact and regulate the blood flow of gastric mucosa[15]. NO can restrain over-production of ET-1 while ET-1 is an active composition that may cause dramatic vasoconstriction. It has been revealed that ET-1 plays a part in the physiopathologic mechanism of gastric mucosal injury. Its effect of causing vasoconstriction can signif i cantly reduce the blood-supply quantity of gastric mucosa and hence weaken the preventive effect[17].
2.6 The level of SOD, NO and MDA in gastric tissues
Table 5 Effect of ABLS administration on the levels of SOD, NO and MDA in gastric tissues of mice with HCl/ethanol-induced gastric mucosal injury
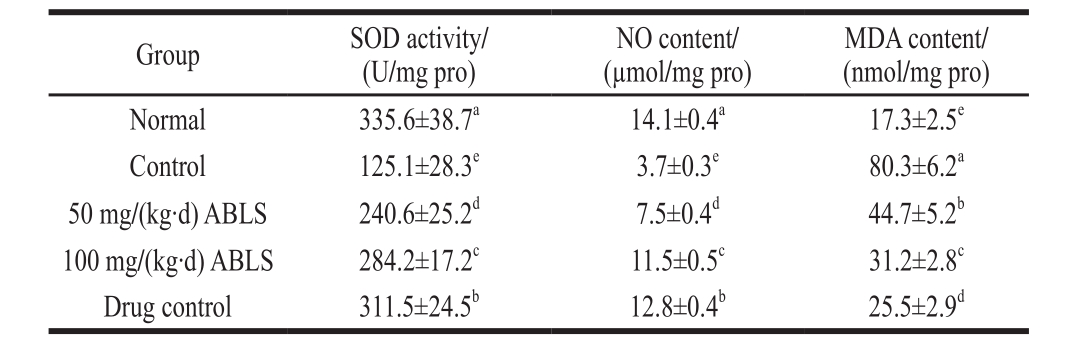
The ABLS could be signif i cantly changed the activities of SOD, NO and MDA (P < 0.05) in mice with HCl/ethanol induced-gastric mucosal injury (Table 5). ABLS treated-mice exhibited higher activities of SOD and NO, lower activity of MDA compared to those of mice in control gastric mucosal injury. ABLS could change the activities of SOD, NO and MDA in stomach close to those of the drug control (ranitidine) and normal groups. Oxygen free radicals can induce lipid peroxidation of gastric mucosa or mucous cells at both overall level and cellular level, generate lipid peroxides like MDA, cause blood stream obstruction and gastric mucosal injury[18]. SOD can directly scavenge free radicals, interrupt their chain reaction and repair cells. It has been revealed that heavy drinking can lead to dramatic decline of SOD activity, weaken the scavenging activity and result in organism injury[19]. NO is a neurotransmitter and messenger molecule. Normally secreted NO can loosen the gastrointestinal smooth muscle and expand blood vessels, inhibit platelet aggregation in gastric mucosal micro-circulation and increase blood fl ow volume of gastric mucosa; it can maintain the integrity of epithelium of gastric mucosa, participate in protection, repair and inhibit gastric acid secretion after gastric mucosal injury; it can also inhibit NF-κB activity, restrain inflammatory response after the injury and shows anti-inflammatory action[20-21]. As NO can protect gastric mucosa by these means, a decreased content of NO in gastric mucosa isanother cause for ethanol-induced gastric mucosal injury. ABLS can effectively prevent drop of NO content in serum which is caused by gastric mucosal injury and hence protect gastric mucosa. MDA is produced because the free radicals attack polyunsaturated fatty acid in biomembrane structure of cells and trigger lipid peroxidation. Its content in serum is indicative of the oxidative damage in cells and tissues. Ethanol intake can increase free radicals by various means and especially by forming MDA[22]. Therefore, MDA level is recommended as the biomarker of alcoholic oxidative stress response.
2.7 The mRNA and protein expression levels of NF-κB, IκB-α, EGF and EGFR in gastric tissues
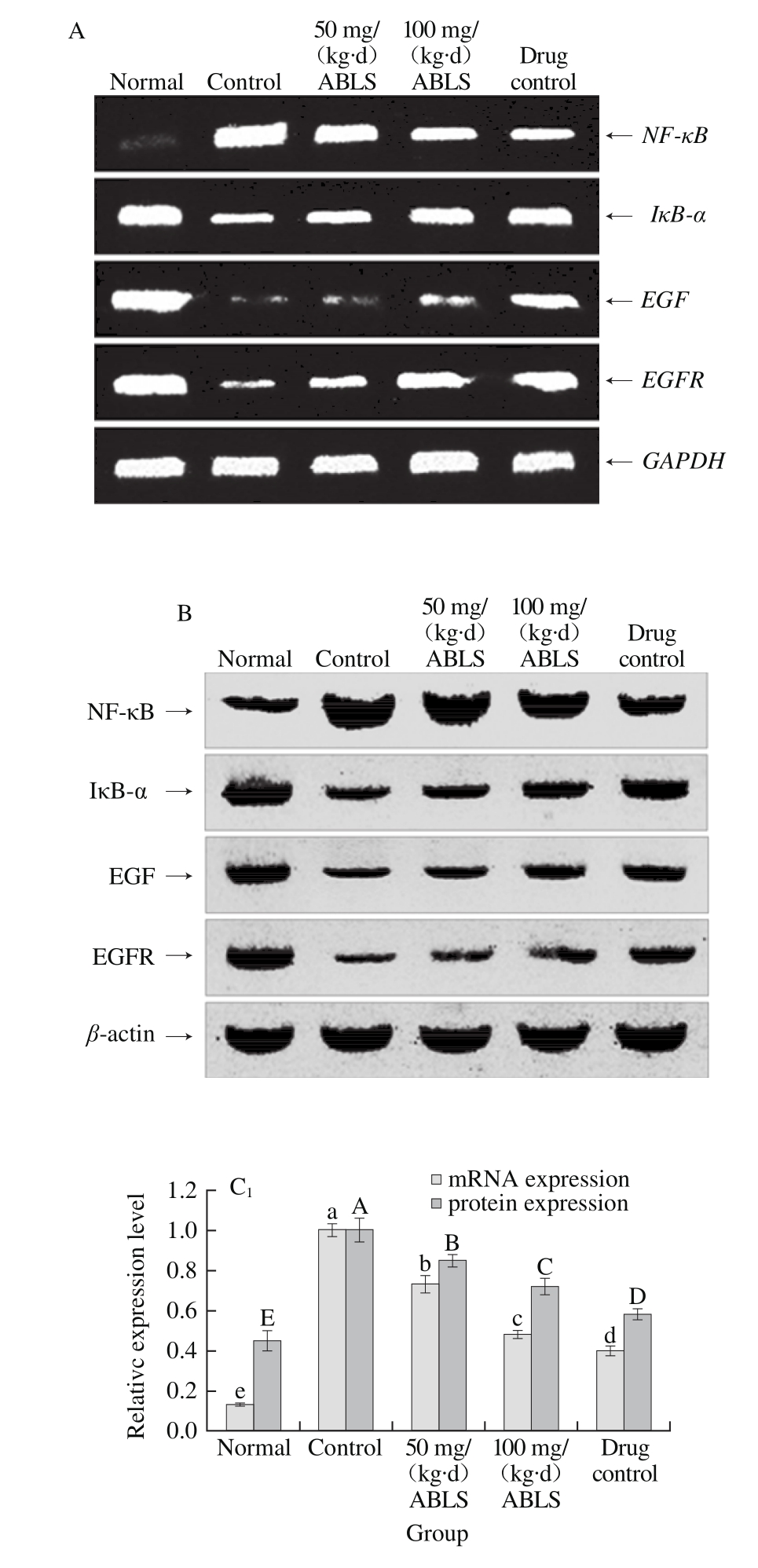
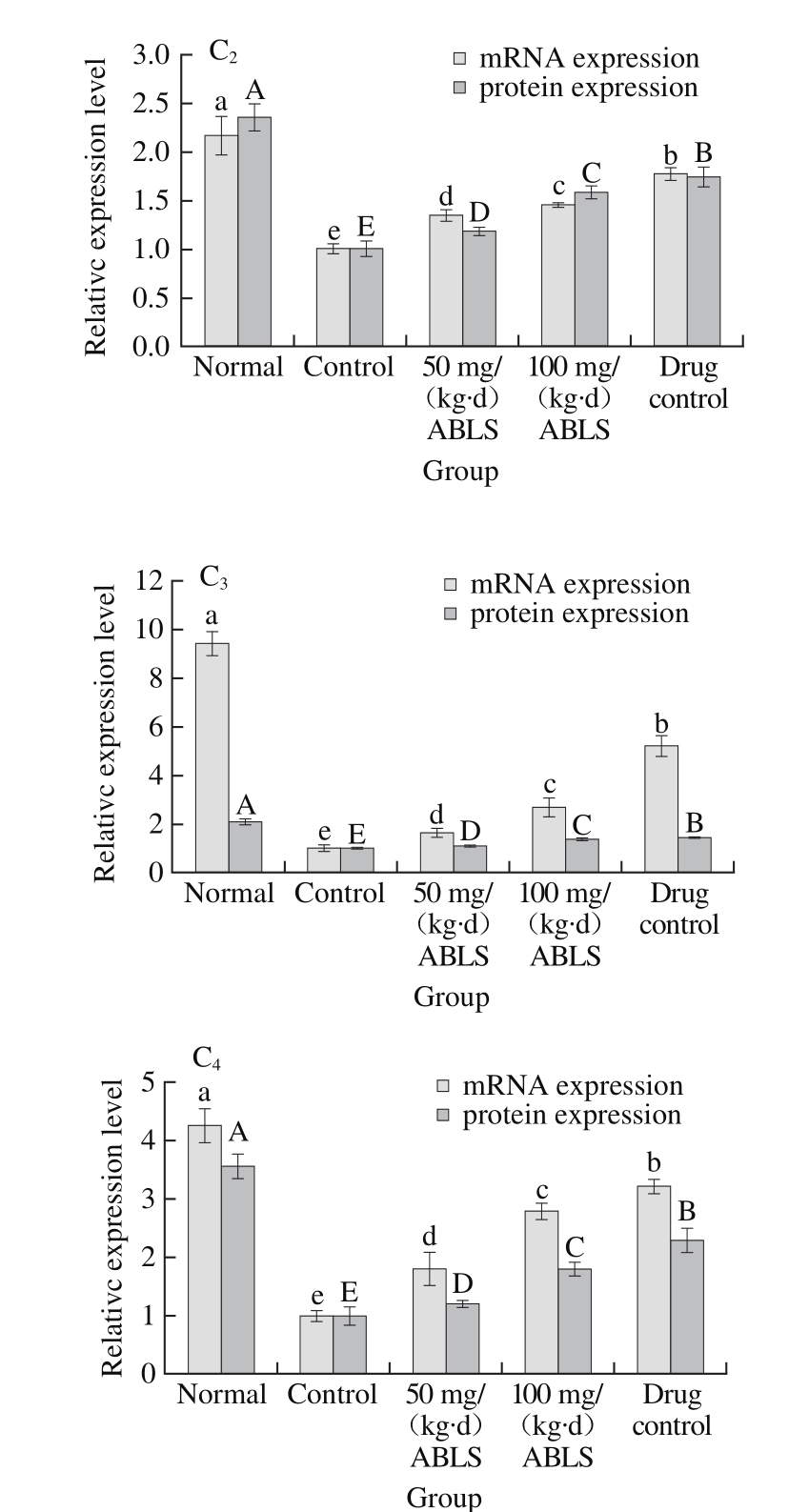
Fig. 2 Effect of ABLS administration on mRNA and protein expression levels of NF-κB, IκB-α, EGF and EGFR in mice with HCl/ethanolinduced gastric mucosal injury
A. mRNA expression of RT-PCR; B. Protein expression of Western blot, Fig. 3 and 4 are the same. C1~4. Relative expression level of NF-κB, IκB-α, EGF and EGFR. Different letters in the same index indicate significant difference (P<0.05).
Gastric mucosal injury could be induced by HCl/ethanol (control group). This response increased mRNA and protein expression levels of NF-κB and reduce mRNA and protein expression levels of IκB-α, EGF, and EGFR in gastric tissues (Fig. 2). ABLS (100 mg/(kg·d)) treatment decreased the mRNA and protein expression levels of NF-κB (0.48 and 0.72 folds of control group), and also increased the expression levels of IκB-α (1.45 and 1.58 folds of control group), EGF (2.70 and 1.35 folds of control group), EGFR (2.79 and 1.80 folds of control group) to those of mice in the control group, and the higher concentration (100 mg/(kg·d)) of ABLS significantly (P < 0.05) raised or reduce more expressions. It has been known that NF-κB and IκB-α play a crucial role in activating vascular endothelial cells[23]. Generally,NF-κB combines with its inhibitory protein IκB-α in an inactive complex form which exists in cytoplasm. Under the stimulus of external signal, IκB-α is phosphorylated and degraded, and dissociated NF-κB is transfers to cell nucleus where it activates relevant genetic expression[10]. This leads to cell metabolic disorder, intracellular acidosis and higher permeability of cellular membrane, makes cells to release a large amount of ET-1 and aggravates gastric injury. ET-1 can accelerate activation of NF-κB, increase its expression and promote degradation of IκB-α. In addition, ET-1 is regulated by NF-κB, so ET-1 secretion of endothelial cells can be inhibited by restraining NF-κB activity. Restraining ET-1 activity by controlling NF-κB expression is also an effective approach to alleviate gastric mucosal injury[24]. Ethanol is destructive to gastric mucosa and sabotages the protective effect of defensive factors in gastric mucosa. The defensive factors mainly include mucosal barrier, mucosal microcirculation and EGF and NO in gastric mucosa[25]. A low EGF level may lead to poor renewal and defense ability of gastric mucosal cells. Ethanol also restrains the reactivity of EGF to gastric mucosal injury, promotes the expression levels of EGF and EGFR in gastric mucosa and hence stimulates proliferation and migration of epithelial cells and accelerates healing of gastric injury[26]. EFG is considered as intermediary factor which regulates ulcer healing. It can signif i cantly inhibit gastric acid secretion by combining with its specif i c receptor (EGFR). It also promotes epitheliosis and tissue reparation, enhances mucosal blood fl ow and plays an important role in protecting and regenerating gastric mucosa. EGF can stimulate proliferation, differentiation and migration of epithelial cells at healing zone at the margin of ulcer, promote formation of microvessels in granulation tissue and regeneration of mucosa and gland[27].
2.8 The mRNA and protein expression levels of nNOS, eNOS and iNOS in gastric tissues
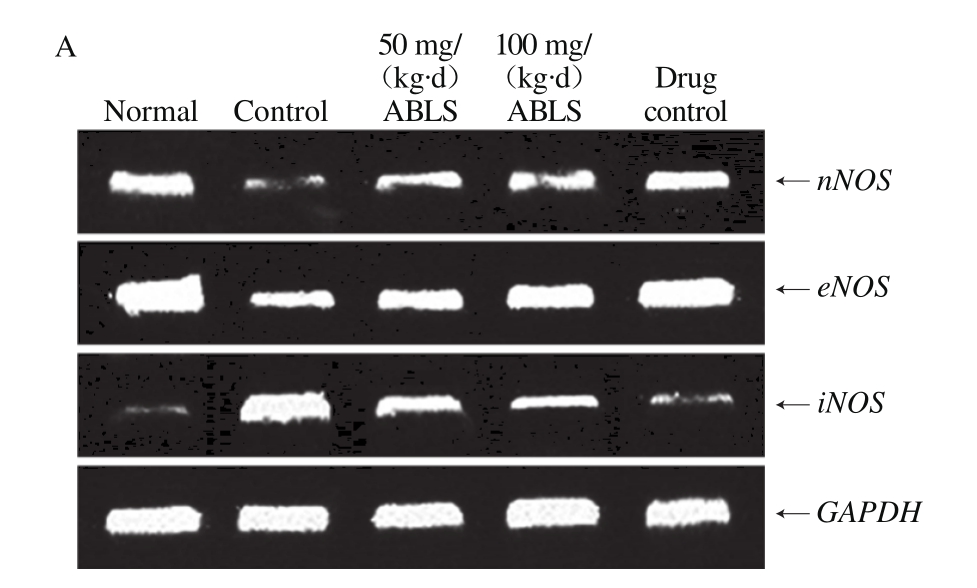
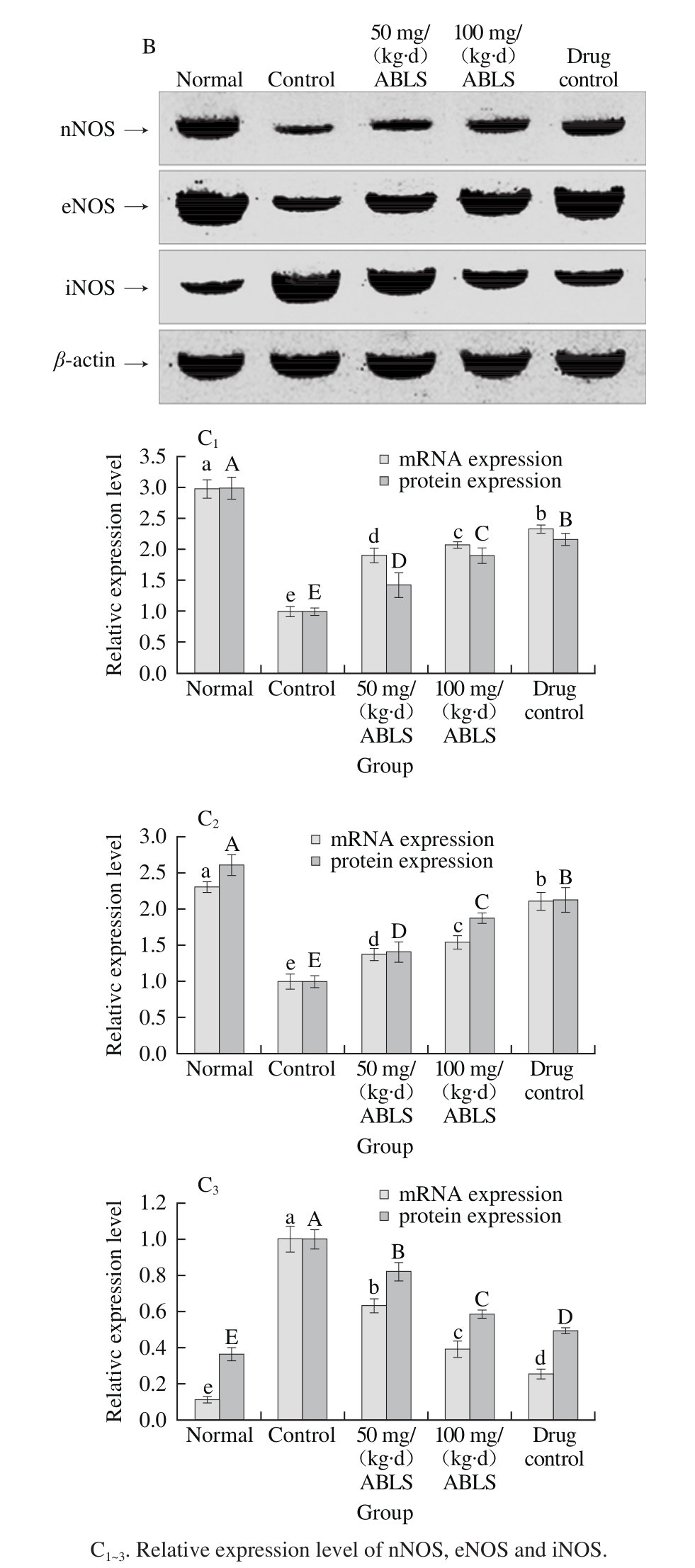
Fig. 3 Effect of ABLS administration on mRNA and protein expression
levels of nNOS, eNOS and iNOS in mice with HCl/ethanol-induced gastric mucosal injury
The mRNA and protein expression levels of nNOS and eNOS in gastric tissues of normal mice were the highest in all group, and these expression levels in mice of control group were lowest (Fig. 3). The iNOS expression in gastric tissue of mice in normal group was lowest and highest in mice of control group. After 100 mg/(kg·d) ABLS treatment, nNOS (2.08 folds mRNA expression, 1.90 folds protein expression)and eNOS (1.54 folds mRNA expression, 1.88 folds protein expression) were raised, and iNOS (0.39 folds mRNA expression, 0.58 folds protein expression) was reduced as compared to those of the control group. These expressions levels in ABLS-treated mice were close to those of mice in the ranitidine and normal groups. NO content in organism is mainly regulated by NOS. NOS in gastric mucosa is divided into two types. One type is inherent NOS which relies on Ca2+(cNOS, including nNOS and eNOS), and the other is inducible NOS which does not rely on Ca2+(iNOS)[28]. cNOS is distributed in nerve cells and endothelial cells. It has stable activity and continuously releases a small amount of NO to protect gastric mucosa. iNOS is not expressed in cells under normal state, but is expressed and continuously releases a large amount of NO when gastric mucosa is injured. Excessive NO can form peroxynitrite with oxygen free radicals and cause lipid peroxidation damage, which is a key cause for gastric mucosal injury[29]. Overinhibition of cNOS and elevation of iNOS activity are closely related with gastric mucosal injury. The injury can be effectively relieved by decelerating the reduced cNOS expression and inhibiting the enhanced iNOS expression[30]. In this study, ABLS helps inhibit gastric mucosal injury.
2.9 The mRNA and protein expression levels of Mn-SOD, Gu/Zn-SOD and CAT in gastric tissues
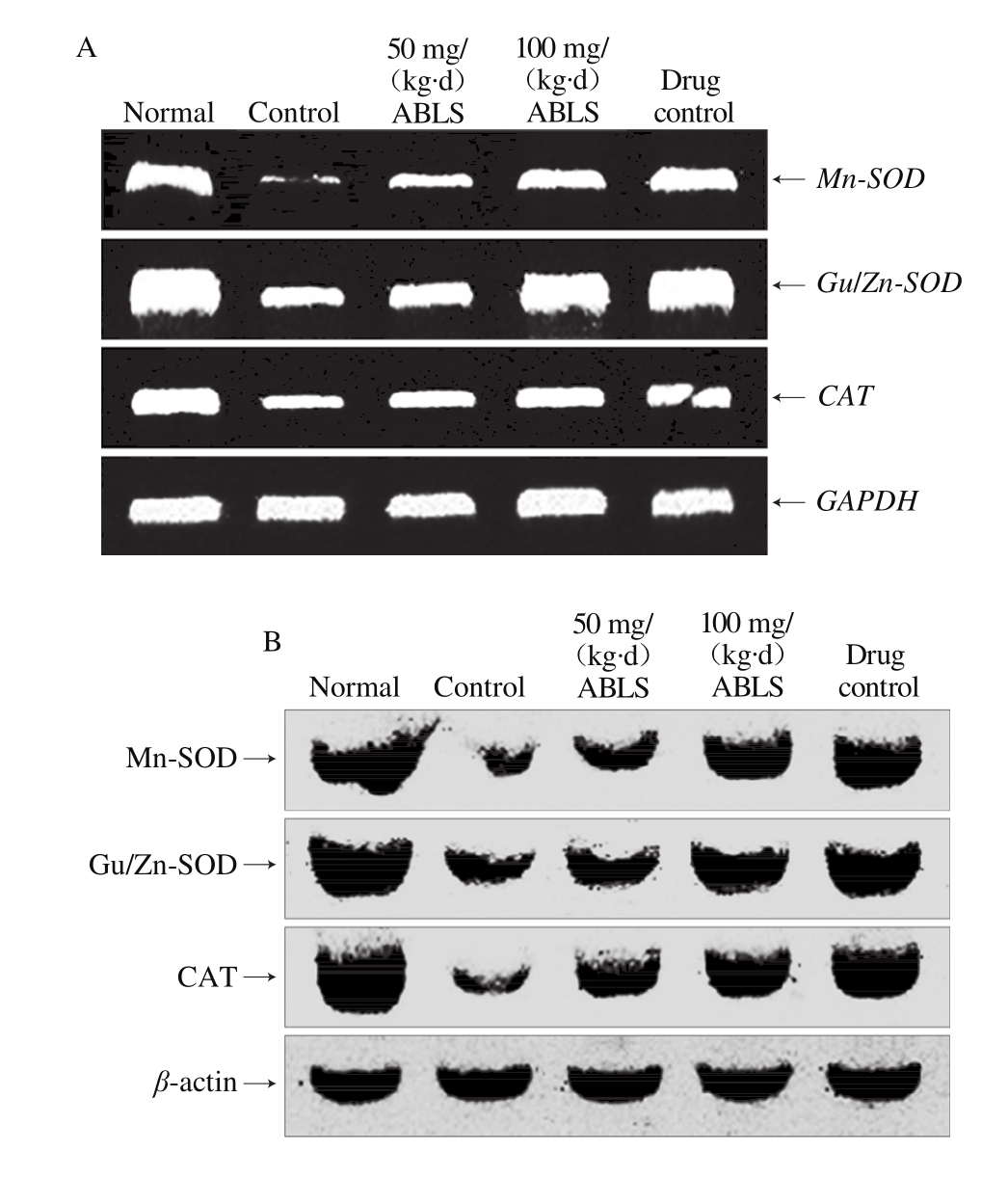
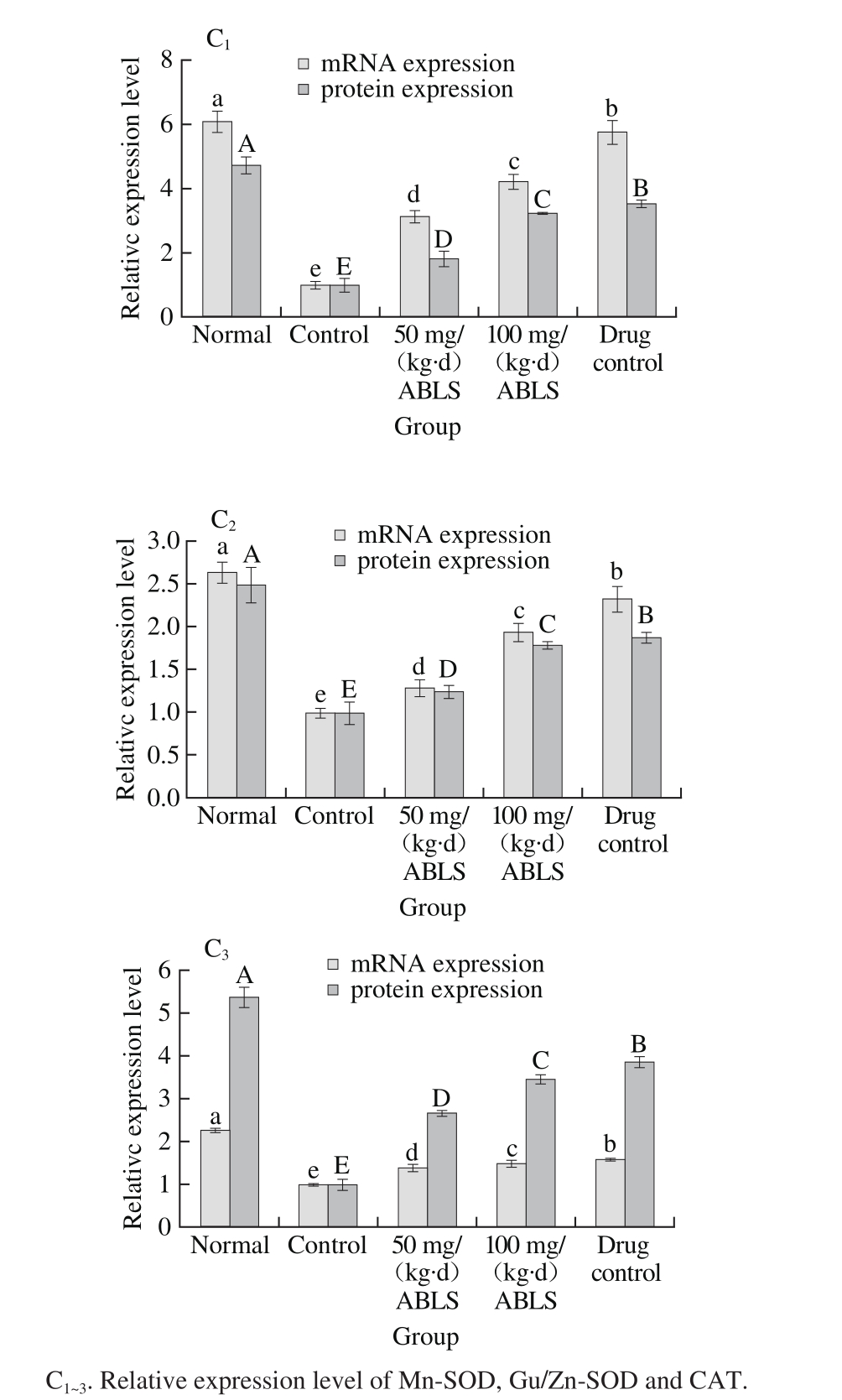
Fig. 4 Effect of ABLS administration on mRNA and protein expression levels of Mn-SOD, Gu/Zn-SOD and CAT in mice with HCl/ethanolinduced gastric mucosal injury
Nomal mice had the highest mRNA and protein expression levels Mn-SOD, Gu/Zn-SOD and CAT, and ABLS-, ranitidinetreated mice showed the higher mRNA and protein expression levels of Mn-SOD (4.22 folds mRNA expression, 3.22 folds protein expression), Gu/Zn-SOD (1.98 folds mRNA expression, 1.55 folds protein expression) and CAT (1.49 folds mRNA expression, 3.44 folds protein expression) than those of mice in control group (Fig. 4). The high concentration ABLS (100 mg/(kg·d)) treatment could raise expression levels of Mn-SOD, Gu/Zn-SOD and CAT as compared to those of mice in the 50 mg/(kg·d) ABLS group. Under the stimulus of ethanol, gastric mucosa released a large amount of oxygen free radicals, among which, OH is highly poisonous[31]. In addition, the content of xanthine dehydrogenase was much higher in the stomach than in the other tissues. When gastric mucosa is stimulated by ethanol, therefore, there is intense oxidative reaction in thestomach. SOD, the most important antioxidant enzyme which resists free radicals, is mainly composed of Cu/Zn-SOD and Mn-SOD. Both Cu/Zn-SOD and Mn-SOD can reduce the oxidation led injury[32]. Hydrogen peroxide is a metabolic waste which injures the organism. To avoid the injury, it must be quickly transformed to other harmless or less poisonous molecules. CAT is usually used to catalyze its decomposition. There is a significant increase in the level of hydrogen peroxide when gastric mucosa is injured. A high level of CAT can alleviate the injury by scavenging hydrogen peroxide[3].
In this study, the influence of ABLS on the mice with gastric mucosal injury was examined with various approaches. It was observed that ABLS reduced the damaged area of gastric mucosa, decreased the gastric juice volume and increased pH value of the gastric juice. ABLS signif i cantly reduced inf l ammatory cytokine IL-6, IL-12, TNF-α and IFN-γ in mouse serum. After the mice were orally given ABLS, the content of MOT and SP in their serum were also controlled while those of SS and VIP were increased. In addition, the contents of SOD, NO and CAT were decreased while those of MDA and ET-1 were increased. Through further test with molecular biological method, it was observed that ABLS enhanced mRNA and protein expression levels of EGF, EGFR, IκB-α, nNOS, eNOS, Mn-SOD, Cu/Zn-SOD and CAT in gastric tissue of the mice, but reduced the expression level of NF-κB and iNOS. These results revealed that ABLS could reduce gastric mucosal injury through various ways by controlling oxidative damage. Moreover, in all tests, with high-concentration ABLS, the relevant indexes of the mouse were closer to those in the mice treated with drug therapy or under its normal state. Therefore, high-concentration ABLS has a better preventive effect to gastric mucosal injury.
[1] HUANG S Y, ZHENG B D. Antioxidant activity of polyphenolic compounds in lotus seed[J]. Journal of Fujian Agriculture and Forestry University (Natural Science Edition), 2010, 39(1): 94-97. DOI:10.13323/j.cnki.j.fafu(nat.sci.).2010.01.022.
[2] GONG H, LU Y Y, CHEN Q B. Summary of lotus plumule research status in China[J]. Popular Science & Technology, 2015, 17(2): 208-209.
[3] PAN J S, HE S Z, XU H Z, et al. Oxidative stress disturbs energy metabolism of mitochondria in ethanol-induced gastric mucosa injury[J]. World Journal of Gastroenterology, 2008, 14(38): 5857-5867. DOI:10.3748/WJG.14.5857.
[4] SHIN I S, JEON W Y, SHIN H K, et al. Banhabaekchulchunma-tang, a traditional herbal formula attenuates absolute ethanol-induced gastric injury by enhancing the antioxidant status[J]. BMC Complementary and Alternative Medicine, 2013, 13: 170. DOI:10.1186/1472-6882-13-170.
[5] 何绍珍, 任建林. 乙醇对胃黏膜作用机制的研究进展[J]. 世界华人消化杂志, 2005, 13(21): 2591-2596. DOI:10.3969/ j.issn.1009-3079.2005.21.016.
[6] GUO R, REN J. Alcohol and acetaldehyde in public health: from marvel to menace[J]. International Journal of Environmental Research and Public Health, 2010, 7(4): 1285-1301. DOI:10.3390/ ijerph7041285.
[7] GIRARD P M, GRAINDORGE D, SMIRNOVA V, et al. Oxidative stress in mammalian cells impinges on the cysteines redox state of human XRCC3 protein and on its cellular localization[J]. PLoS ONE, 2013, 8(10): e75751. DOI:10.1371/journal.pone.0075751.
[8] RACKOVA L, OBLOZINSKY M, KOSTALOVA D, et al. Free radical scavenging activity and lipoxygenase inhibition of Mahonia aquifolium extract and isoquinoline alkaloids[J]. Journal of Inf l ammation, 2007, 4: 15. DOI:10.1186/1476-9255-4-15.
[9] LI J, XIA Y B. Research progress on healthy function with alkaloid in embryo of lotus seed[J]. Cereals & Oils, 2010, 2010: 40-43.
[10] LI G J, SUN P, WANG R, et al. Preventive effect of polysaccharide of Larimichthys crocea swim bladder on reserpine induced gastric ulcer in ICR mice[J]. Korean Journal of Physicology & Pharmacology, 2014, 18(2): 183-190. DOI:10.4196/kjpp.2014.18.2.183.
[11] ZHAO X, WANG Q, QIAN Y. Ilex kudingcha CJ Tseng (Kudingcha) prevents HCl/ethanol-induced gastric injury in Sprague-Dawley rats[J]. Molecular Medicine Reports, 2013, 7(5): 1613-1616. DOI:10.3892/ mmr.2013.1402.
[12] QIAN Y, LI G J, ZHU K, et al. Effect of resistant starch on HCl/ ethanol-induced gastric injury in rats[J]. Journal of the Korean Society for Applied Biological Chemistry, 2013, 56(5): 613-619. DOI:10.1007/ s13765-013-3143-4.
[13] CHEN S C, ZHU K, WANG R, et al. Preventive effect of polysaccharides from the large yellow croaker swim bladder on HCl/ethanol induced gastric injury in mice[J]. Experimental and Therapeutic Medicine, 2014, 8(1): 316-322. DOI:10.3892/etm.2014.1712.
[14] VIGNALI1 D A A, KUCHROO V K. IL-12 family cytokines: immunological playmakers[J]. Nature Immunology, 2012, 13(8): 722-728. DOI:10.1038/ni.2366.
[15] DU Y, ZHAO W, LU L, et al. Study on the antiulcer effects of Veronicastrum axillare on gastric ulcer in rats induced by ethanol based on tumor necrosis factor-α (TNF-α) and endothelin-1 (ET-1)[J]. Asian Pacif i c Journal of Tropical Biomedicine, 2013, 3(12): 925-930. DOI:10.1016/S2221-1691(13)60180-X.
[16] ZHOU Y L, WANG R, FENG X, et al. Preventive effect of insect tea against reserpine-induced gastric ulcers in mice[J]. Experimental and Therapeutic Medicine, 2014, 8(4): 1318-1324. DOI:10.3892/ etm.2014.1859.
[17] LOPEZ-BELMONTE J, WHITTLE B J, MONCADA S. The actions of nitric oxide donors in the prevention or induction of injury to the rat gastric mucosa[J]. British Journal of Pharmacology, 1993, 108(1): 73-78.
[18] AL-ABBASI F A. Acrylonitrile-induced gastric toxicity in rats: the role of xanthine oxidase[J]. Medical Science Monitor, 2012, 18(6): 208-214. DOI:10.12659/MSM.882896.
[19] PENG C, WANG X, CHEN J, et al. Biology of ageing and role of dietary antioxidants[J]. BioMed Research International, 2014, 2014: 831841. DOI:10.1155/2014/831841.
[20] ZHANG Y, PATERSON W G. Nitric oxide contracts longitudinal smooth muscle of opossum oesophagus via excitation-contraction coupling[J]. Journal of Physiology, 2001, 536(Pt 1): 133-140. DOI:10.1111/j.1469-7793.2001.00133.x.
[21] JIN Q H, SHEN H X, WANG H, et al. Curcumin improves expression of SCF/c-kit through attenuating oxidative stress and NF-κB activation in gastric tissues of diabetic gastroparesis rats[J]. Diabetology & Metabolic Syndrome, 2013, 5: 12. DOI:10.1186/1758-5996-5-12.
[22] MAGIEROWSKI M, JASNOS K, SLIWOWSKI Z, et al. Exogenous asymmetric dimethylarginine (ADMA) in pathogenesis of ischemiareperfusion-induced gastric lesions: interaction with protective nitric oxide (NO) and calcitonin gene-related peptide (CGRP)[J]. International Journal of Molecular Sciences, 2014, 15(3): 4946-4964. DOI:10.3390/ijms15034946.
[23] MAJDALAWIEH A, RO H S. Regulation of IκBα function and NF-κB signaling: AEBP1 is a novel proinflammatory mediator in macrophages[J]. Mediators of Inflammation, 2010, 2010: 823821. DOI:10.1155/2010/823821.
[24] YADAV V R, PRASAD S, SUNG B Y, et al. The role of chalcones in suppression of NF-κB-mediated inflammation and cancer[J]. International Immunopharmacology, 2011, 11(3): 295-309. DOI:10.1016/j.intimp.2010.12.006.
[25] BOGDANOVA O V, KOT L I, LAVROVA K V, et al. Modulation of protein tyrosine phosphorylation in gastric mucosa during re-epithelization processes[J]. World Journal of Biological Chemistry, 2010, 1(11): 338-347. DOI:10.4331/wjbc.v1.i11.338.
[26] YANG Z B, YAN J, ZOU X P, et al. Enhanced expression of epidermal growth factor receptor gene in gastric mucosal cells by the serum derived from rats treated with electroacupuncture at stomach meridian acupoints[J]. World Journal of Gastroenterology, 2006, 12(34) : 5557-5561. DOI:10.3748/wjg.v12.i34.5557.
[27] OH T Y, AHN B O, JANG E J, et al. Accelerated ulcer healing and resistance to ulcer recurrence with gastroprotectants in rat model of acetic acid-induced gastric ulcer[J]. Journal of Clinical Biochemistry and Nutrition, 2008, 42(3): 204-214. DOI:10.3164/jcbn.2008030.
[28] FENG C J. Mechanism of nitric oxide synthase regulation: electron transfer and interdomain interactions[J]. Coordination Chemistry Reviews, 2012, 256(3/4): 393-411. DOI:10.1016/j.ccr.2011.10.011.
[29] HAJREZAIE M, GOLBABAPOUR S, HASSANDARVISH P, et al. Acute toxicity and gastroprotection studies of a new schiff base derived copper (II) complex against ethanol-induced acute gastric lesions in rats[J]. PLoS ONE, 2012, 7(12): e51537. DOI:10.1371/ journal.pone.0051537.
[30] LIU Y, LINGSHAN G, CUI Y, et al. A preliminary study on protective effect of L-citrulline against ischemia-reperfusion induced gastric mucosal lesions in rat[J]. Indian Journal of Pharmacology, 2012, 44(1): 31-35. DOI:10.4103/0253-7613.91863.
[31] LIU J L, DU J, FAN L L, et al. Effects of quercetin on hyperproliferation of gastric mucosal cells in rats treated with chronic oral ethanol through the reactive oxygen species-nitric oxide pathway[J]. World Journal of Gastroenterology, 2008, 14(20): 3242-3248. DOI:10.3748/wjg.14.3242.
[32] FUKAI T, USHIO-FUKAI M. Superoxide dismutases: role in redox signaling, vascular function, and diseases[J]. Antioxidants & Redox Signaling, 2011, 15(6): 1583-1606. DOI:10.1089/ars.2011.3999.
巴莲莲子生物碱减弱盐酸/乙醇诱导的小鼠胃黏膜损伤研究
易若琨1,2,冯 霞1,2,李贵节1,2,彭德光3,母健菲1,2,符成刚1,2,赵 欣1,2,*
(1.重庆第二师范学院 重庆市功能性食品协同创新中心,重庆市功能性食品工程技术研究中心,功能性食品研发重庆市工程实验室,重庆 400067;2.重庆第二师范学院生物与化学工程学院,重庆 400067;3.重庆市巴莲品种选育及深加工企业工程技术研究中心,重庆 400041)
摘 要:本研究的目的是探讨巴莲莲子生物碱对盐酸/乙醇诱导的胃黏膜损伤的作用并对其机制进行研究。小鼠被巴莲莲子生物碱(alkaloids from Ba lotus seed,ABLS)灌胃处理后用盐酸/乙醇诱导胃黏膜损伤,小鼠的血清细胞因子及其他指标采用试剂盒进行测定,同时用逆转录聚合酶链式反应和western blot对胃组织进行检测。100 mg/(kg·d)的ABLS处理小鼠的胃黏膜损伤面积、胃损伤抑制率、胃液分泌量和pH值分别为1.13 mm2、84.5%、0.52 mL和3.3。ABLS作用的小鼠血清白细胞介素(interleukin,IL)-6、IL-12、肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)、γ-干扰素(interferon-γ,IFN-γ)、胃动素(motilin,MOT)、P物质(substance P,SP)、内皮素-1(endothelin-1,ET-1)水平低于对照组小鼠,而生长抑素(somatostatin,SS)、血管活性肠肽(vasoactiveintestinal peptide,VIP)水平高于对照组。ABLS作用小鼠胃组织比对照组小鼠具有更高的超氧化物歧化酶(superoxide dismutase,SOD)活性和更低的MDA含量。相比对照组ABLS处理还可以上调胃组织中核因子κB抑制蛋白α(inhibitor kappaB-α,IκB-α)、表皮生长因子(epidermal growth factor,EGF)、表皮生长因子受体(epidermal growth factor receptor,EGFR)、神经元型一氧化氮合酶(euronal nitric oxide synthase,nNOS)、内皮型一氧化氮合酶(endothelial nitric oxide synthase,eNOS)、Mn-SOD、Gu/Zn-SOD、过氧化氢酶(catalase,CAT)的mRNA和蛋白表达,同时下调核因子κB(nuclear factor kappaB,NF-κB)、诱导型一氧化氮合成酶(inducible nitric oxide synthase,iNOS)表达。这项研究表明ABLS处理可减轻胃黏膜损伤,并且这种作用是来自ABLS的抗氧化能力。
关键词:巴莲;抗炎;生物碱;抗氧化
References:
收稿日期:2016-12-28
基金项目:重庆高校创新团队建设计划资助项目(CXTD201601040);重庆市工程技术研究中心建设项目(cstc2015yfpt_gcjsyjzx0027);重庆第二师范学院科研项目一般项目(KY201523B)
作者简介:易若琨(1989—),女,助教,硕士,研究方向为食品化学与营养学。E-mail:yiruokun1214@hotmail.com
中图分类号:TS272.5
文献标志码:A
文章编号:1002-6630(2017)15-0221-11
引文格式:
*通信作者:赵欣(1981—),男,教授,博士,研究方向为食品化学与营养学。E-mail:zhaoxin@cque.edu.cn
DOI:10.7506/spkx1002-6630-201715036
YI Ruokun, FENG Xia, LI Guijie, et al. Alkaloids from Ba lotus seeds (Nelumbo nucifera Gaertn) attenuates HCl/ethanol induced gastric mucosal injury in ICR mice[J]. 食品科学, 2017, 38(15): 221-231.
DOI:10.7506/spkx1002-6630-201715036. http://www.spkx.net.cn
YI Ruokun, FENG Xia, LI Guijie, et al. Alkaloids from Ba lotus seeds (Nelumbo nucifera Gaertn) attenuates HCl/ethanol induced gastric mucosal injury in ICR mice[J]. Food Science, 2017, 38(15): 221-231. (in English with Chinese abstract) DOI:10.7506/spkx1002-6630-201715036. http://www.spkx.net.cn