MING Zhu, CHEN Qingsen*, ZHANG Lei, YAN Yali, ZHAO Pei
(Tianjin Key Laboratory of Food Biotechnology, College of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China)
Abstract:T he present study aimed to evaluate the effi cacy of phytosterol (PS), phytosterol ester (PSE) and dairy products supplemented with PS and/or PSE in reducing the risk of cardiovascular and cerebrovascular diseases for the purpose of providing a scientific basis for guiding the practical use of plant-derived phytochemicals and Yangxin dairy (YXD), a commercial dairy product supplemented with both PS and PSE. SD rats were randomly divided into 9 groups with 10 rats in each group. The rats in the normal control and model groups were fed a normal diet and a high fat diet, respectively and then administrated by gavage with 0.9% normal saline, while those in the PS, PSE, YXD and placebo groups were given a high fat diet and gavaged with PS at 23.6 and 47.2 mg/mL, PSE at 23.6 and 47.2 mg/mL, YXD at 30 and 60 g/250 mL and peanut oil, respectively. After 4 consecutive weeks of administration, all the rats were sacrifi ced for the collection of blood and visceral tissue samples. The results showed that total serum cholesterol levels in the rats in the PS, PSE and YXD groups signifi cantly decreased and serum high-density lipoprotein cholesterol levels increased in a dose-dependent manner compared with the model group. These treatments also caused a reduction of triglyceride, total antioxidant capacity and malonaldehyde levels, although the effect was unstable. The same trends were also seen for the corresponding indicators in liver and pathological sections. PSE and YXD had better effi cacy in reducing the risk of cardiovascular and cerebrovascular diseases. Furthermore, YXD had higher potential to reduce the risk of cardiovascular diseases and protect the liver and blood vessels.
Key words:phytosterols; phytosterol ester; cardiovascular and cerebrovascular diseases; cholesterol-reducing
引文格式:
MING Zhu, CHEN Qingsen, ZHANG Lei, et al. Evaluation of the effi cacy of phytosterol and phytosterol-supplemented dairy products in reducing the risk of cardiovascular and cerebrovascular diseases[J]. 食品科学, 2017, 38(17): 174-183. DOI:10.7506/spkx1002-6630-201717029. http://www.spkx.net.cn
MING Zhu, CHEN Qingsen, ZHANG Lei, et al. Evaluation of the effi cacy of phytosterol and phytosterol-supplemented dairy products in reducing the risk of cardiovascular and cerebrovascular diseases[J]. Food Science, 2017, 38(17): 174-183. (in English with Chinese abstract) DOI:10.7506/spkx1002-6630-201717029. http://www.spkx.net.cn
With the increasing of aging population and younger trend of cardiovascular and cerebrovascular diseases (CCDs), more and more people have begun to concern and pay attention to the hot topic of “how to prevent and cure CCD”. According to the statistic data of World Health Organization (WHO), CCDs is “the number 1 killer” that causes substantial harm on the health of the global population and result in death. Each year, approximately 15 million people in the world died off due to CCDs a ccounting for more than 50% of the total death caused by all the diseases[1]. According to the China Public Health Statistical Yearbook in 2012, malignant cancers, cardiovascular and cerebrovascular diseases are the first three leading causes of death in the people living in the urban and rural areas, among which, the combination of the death cases caused by cardiovascular diseases and cerebrovascular diseases accounted for more than 40% of the total death cases, being ranked the fi rst place among all the causes of death. The unhealthy living styles can lead to gradual increase of lipids and cholesterol-derived substances levels in human body, causing the deposit on the blood vessel wall of low-density lipoprotein cholesterol (LDL-C) after oxidation. For a long run, this deposit of lipids will cause the occlusion of blood capillary, leading to the occurrence of CCDs. Clinically, majority methods currently used for the reduction of blood cholesterol level require the strict control of the intake of food and drink, and also rely on the use of medicinal drugs. However, the side effects of these drugs largely limit their applications[2]. It would certainly be the best way to reduce the cholesterol levels inside the body by the intake of the biologically active substances present in foods and drinks. In fact, many studies on the roles of PS in the prevention and therapeutic treatment of CCDs have been conducted in both China and foreign countries and a series of research results have been obtained. For instance, Ji Guozhi et al.[3]investigated the roles of α-linoleic acid (α-L A) and PSE-containing milk in reducing the levels of blood lipids in a Sprague Dawley (SD) rat model and found that there were signifi cant differences in the levels of total cholesterol (TC), triglyceride (TG) and high density lipoprotein cholesterol (HDL-C) between the model group and control group. Thus, they thought that PSE compound milk may assistant blood lipid reduction. A study by Chen Maobin et al.[4]indicated that PSE significantly reduced the blood levels of TC and LCL-C in a hyperlipidemia mice model and reduced arteriosclerosis index (AI) and thus, played a good role in prevention of hyperlipidemia and arteriosclerosis symptoms. Another study showed that PSE could effectively reduce the levels of TC and LDL and increase the level of HDL in volunteers who taking milk powder that contains phytosterol ester for two months[5]. In addition, PSE also significantly reduced the liver weight, liver index and the hepatic levels of TC and TG and thus, effectively alleviated the pathogenesis of the fatty diseases. The study by Guo Yubao et al.[6]found that PSE was capable of significantly reducing the blood levels of TC and LDL-C in mice in a relatively short time. Another study by Thornton found that dietary supplement with PS and VC reduced the body mass accumulation and altered food transit time in obesity mouse[7]. Tikkanen et al.[8]gave 1.25–5.00 g non-esterified PS daily to patients with hyperlipidemia for consecutive 15 weeks and found that non-esterified PS significantly reduced the serum levels of TC and LDL-C in these patients. The study by Hallikainen et al.[9]also found that the daily intake of 1–2 g PS reduced the serum cholesterol level by 10%–15%. In a short-term cross-sectional study, it was found that when the studied subjects were given 3 g lecithin-emulsified stanol daily for consecutive 3 days, the cholester ol absorption was reduced by 40%[10]. Blair et al.[11]gave PSE as a food additive to the studied subjects in the experimental group and found that when the studied subjects took in 5.1 g PSE daily. After 8 weeks, the levels of TC and LDL-C were reduced by 12% and 17% , respectively, as compared to those of the subjects in the placebo-group. The study by Nguyen et al.[12]indicated that daily intake of 24 g liniment oil containing 2–3 g PSE reduced the serum levels of TC and LDL-C by about 6.5% and 10.1%, respectively. Other related studies indicatedthat as biologically active substances, PS and its derivative products had many important physiological functions including anti-arteriosclerosis, prevention of non-alcoholic fatty liver disease[13], reduction of cholesterol absorption[14], anti-oxidation[15]as well as unique nutritional characteristics. Thus, they have been widely applied as healthy foods and medicinal drugs, playing signifi cant roles in prevention and therapeutic treatment of CCDs[16].
In this study, healthy male SD rats were randomly divided into 9 groups and they were perfused via perturbation with PS, PSE and the dairy product containing PS E as an additive at corresponding concentrations. The serum levels of several indexes including TC, TG, HDL-C, LDL-C, malonaldehyde (MDA), and total antioxidant capacity (T-AOC) were measured. The pathological sections of liver, heart and arcus aortae were prepared and observed and the changes in the levels of indexes such as the hepatic TC, TG and MDA were monitored. This study was conducted to investigate the role of PS, PSE and their corresponding dairy products in the improvement and prevention of CCDs, aiming to provide the theoretical basis for the consumption and practices of dietary-derive d biologically active substances in a scientifi c way.
1.1 Materials and reagents
1.1.1 Experimental animals and their feeds
Eight weeks old and healthy male SD rats (specific pathogen free (SPF) grade) with body weights of (180 ± 10) g, and basic, high fat-diet (containing basic diet plus 1 g cholesterol, 0.2 g sodium cholate, 10 g pork oil, 10 g sucrose and 10 g egg yolk powder) were purchased from The Experimental Animal Center of Military Medical Institute of People’s Liberation Army (Beijing, China).
1.1.2 Reagents
TC kit, HDL-C kit, TG kit, MDA kit, and T-AOC kit Nanjing Jiancheng Bioengineering Institute (Nanjing, China); PS (a purity of 95%) Xi’an Healthful BioTech Co. Ltd.; PSE, Yangxin dairy (YXD) products Anonymous Company; 5S-pressed first grade peanut oil Shandong Luhua Group Co. Ltd. (Yantai, China).
1.2 Instruments and equipments
FDU-810 vacuum freeze drier Rikakikai Co. Ltd. (Tokyo, Japan); 3-18k centrifuge Sigma (St. Luis, USA); MDF-U32V (N)-80 ℃ freezer Sanyo Electric Co. (Japan); JA2003-analytical balance Shanghai Qianjin Electronic Equipment Co. Ltd. (Shanghai, China).
1.3 Experimental methods
1.3.1 Grouping and raising experimental animals
The experimental animals were raised in Tianjin Municipal Experimental Animal Center. The experimental protocols were approved by The Animal Care and Use Committee of Tianjin City (Permission No: SYXK (Jin) 2011-008; Followed Guidelines NIH Publications No. 8023, 1978). The animals were adaptively fed with the basic diet for one week with free access to foods. The amounts of the weekly intake of diet and body weight were recorded. The animals were randomly divided into 9 groups with 10 animals in each group. At the start of experiments, the animals in various experimental groups were fed with the corresponding normal diet and high fat diet and then were perfused with 0.9% physiological saline, PS (23.6, 47.2 mg/mL), PSE (23.6, 47.2 mg/mL), YXD (30, 60 g/250 mL) and peanut oil correspondingly, lavage dose was 0.25 mL/100 g bw. According to the US FDA rules, PS products with “healthy”label must strictly follow such that each food contains at least 0.65 g PSE or 1.7 g plant stanol ester[17]. In this study, the doses for PS and PSE were chosen based on it; the PS1 and PSE1, PS2 and PSE2 doses were 23.6 and 47.2 mg/mL; the main nutrients of YXD included PSE (252 mg/100 g), protein (3.3 g/100 mL), fat (3.3 g/100 mL)), carbohydrate (4.5 g/100 mL)), and sodium (75 mg/100 g). YXD1 was 30 g/250 mL and YXD2 was 60 g/250 mL. The liquid milk was turned into solid powder by freeze-drying. YXD was prepared according to PSE concentrations.
1.3.2 Preparation of rat histological samples
According to the research scheme, 2, 4 and 6 weeks after intragastric administration with the PS, PSE, YXD and peanut oil, blood samples were drawn from the epicanthic fold of eye socket of the rats. They were fasted for 12 h before blood drawing. After being settled for 30 min, the blood samples were centrifuged at 3 000 r/min at 4 ℃ for 15 min. The serum samples were stored in a -80 ℃ freezer for subsequent analyses. Six weeks after administration with phytosterols, the rats were fasted for 12 h and blood samples were drawn. They were then sacrifi ced by cervical dislocation. The liver, heart, spleen, pancreas, kidney, perinephrit fat and testicular fat were all taken and weighted, and were analyzed for organ indexes. 5 g of liver sample was taken and put into a frozen tube for subsequent analysis of the relevant indexes. Arcus aortae was separated from the upper terminal of the heartand stored in neutral formalin fixative solution for fixation together with heart and an aliquot of liver for the subsequent preparation of histological sections.
1.3.3 Preparation of hepatic homogenates
Liver tissues were cut into small pieces and put into glass homogenizer tube and homogenized at the ratio of liver: physiological saline of 1:10 (m/V). The tissue homogenate was prepared into a 10 g/100 mL homogenate and the centrifuged at 3 000-4 000 r/min for 10-15 min. The supernatant was saved as the liver tissue homogenates. The levels of TC, TG and MDA present in the homogenates were measured.
1.3.4 Measurement and calculation of various indexes 1.3.4.1 Measurement the serological indexes
The changes in the serological indexes (TC, TG, HDL-C, MDA content and T-AOC) in peripheral blood samples were measured with corresponding kits.
1.3.4.2 Measurement and calculation of the organ indexes
The liver, heart, spleen, pancreas, kidney, perinephrit fat and testicular fat were all collected from rats after dissection. These organs were rinsed with phosphate buffer solusion (PBS) and the extra liquid was absorbed with filter paper. These organs were placed in the analytical balance and weighted individually. The organ indexes were calculated according to the equations below.
1.3.4.3 Observation of histological sections
In order to prepare the histological sections with high quality, the preparation of histological sections was conducted according to the procedures described by Pan Lin[18].
1.4 Data treatment and image analysis
Data reorganization and analyses of all the experimental data and quantitative analysis were expressed as ± s. SPSS 19.0 software was used for One-way analysis of variance. The difference between groups with P < 0.05 was regarded as statistically signifi cant.
2.1 The effects of PS, PSE and YXD on the organ indexes of rats
The changes in the organ indexes of various organs of rats in various groups were monitored according to the methods described in section 1.3.4.2. The results were presented in Table 1.
Table 1 Effects of PS, PSE and YXD on organ indexes of rats with hyperlipidemia
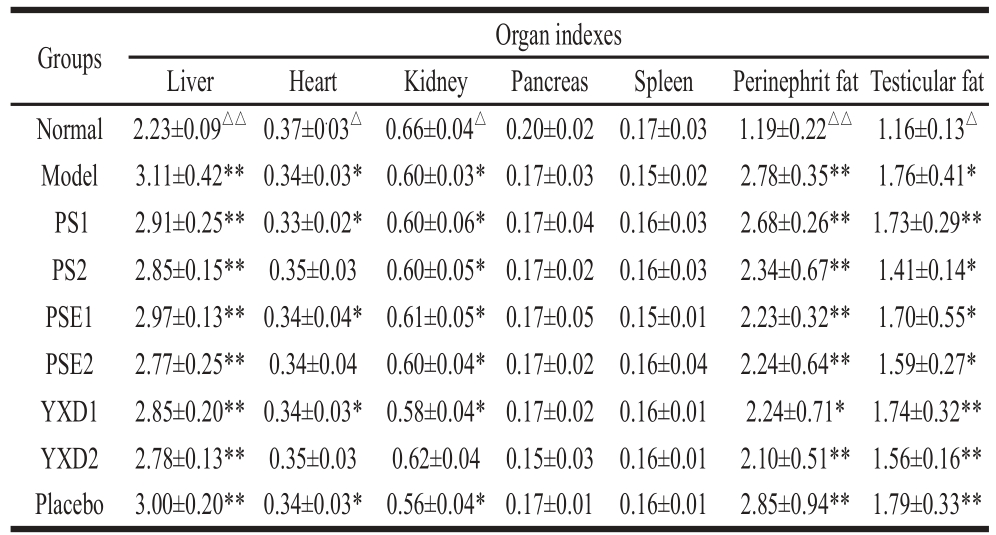
Notes: *. Compared to normal group, a signifi cant difference was indicated
(P<0.05); **. Compared to normal group, an extremely difference was indicated (P<0.01). △. Compared to model group, the signifi cant difference was indicated (P<0.05); △△. Compared to normal group, an extremely difference was indicated (P<0.01).
From Table 1, it can be seen that compared to normal group, there were extremely significant differences in liver index of rats among various groups (P < 0.01), indicating that the intake of high fat-diet can significantly increase liver weight. It can leads to the excess intake of cholesterol, which cannot be metabolized and is deposited in liver, resulting the increase liver weights. While the liver index of the rats in the groups given phytosterols was reduced to certain extents, the effects of these phytosterols on liver index were not significant. However, the experimental data did show that there were clear dose-response relationships for the effects of these phytosterols on liver index in various experimental groups. For the heart index of the rats in various groups, compared to that in the normal group, there was significant differences in heart index of the rats in the group fed with high fat-diet and the groups given low doses of phytochemicals (P < 0.05), indicating that feeding rats with high fat-diet causes significant effects on heart index. There were no significant differences in the heart index of rats between the normal group and the groups given with high doses of PS and PSE (P > 0.05), indicating that these phytosterols have certain effects on reducing the heart index. There were signifi cant differences in the kidney index of rats between the normal group and all groups (P < 0.05) except YXD2 groups, indicating that high dose of YXD diet has certain protecting and alleviating effects on kidney.
2.2 The serum indexes of rats in different groups
It can be seen from Fig. 1, 2 weeks after administration of phytosterols, there was a signifi cant difference in HDL-C and TG content between the normal group and the model group (P < 0.05), indicating that the HDL-C, TG levels were significantly changed in rats feeding with high-fatdiet. After 2 weeks, there were significant (P < 0.05) or extremely significant (P < 0.01) differences in TG, LDL-C levels between the model groups and all experimental groups, the model groups and normal group, indicating that after being administrated to the rats, PS, PSE and YXD can cause significant effects on TG and LDL-C levels in a relatively short time.

Fig. 1 Changes in serum parameter levels among various groups of rats
Four weeks after treatment, compared to that in the normal group, the serum TC level in the model group was extremely increased (P < 0.01), indicating that the high fat diet-model has been successfully established. There were significant (P < 0.05) and extremely significant (P < 0.01) differences in TG, HDL-C and LDL-C levels of SD rats existed between the model group and all the experimental groups administrated with both low and high doses, indicating that intragastric administration of SD rats with PS, PSE and YXD for 4 weeks can signifi cantly reduce TG, LDL-C levels and increase the HDL-C levels. Compared with that of the model groups, there were extremely significant changes in serum TC levels of the rats in the groups given drugs (P < 0.01) after 6 weeks treatments.
It can be seen that with extending timing of administration with phytosterols, the reducing effects on TC level in various experimental groups were gradually enhanced. Among which, the reducing effects in group PSE2 and YXD2 were the most signifi cant ones at 6 weeks. While comparing the TG level in model group with those in the groups given phytochemicals, extremely significant differences (P < 0.01) existed between model group and all the drug-given groups regardless the doses, indicating that intragastric administration of SD rats with PS, PSE and YXD for 6 weeks can reduce TG levels to certain extents but the TG levels in SD rats in drug-given groups are still much higher than that in the control group.
Multiple comparisons between the groups that were given with the same phytosterol but at two different doses were conducted. At the second week, there were not significant differences between two groups. After 4 and 6 weeks, there were extremely significant differences in all the groups given these phytosterols with either lower or higher doses (P < 0.01), indicating that the effects of PS, PSE and YXD in effecting TC, HDL-C and LDL-C level all largely depend on their doses and that these dose-response relationships were enhanced with extending timing of administration. It can be even seen from these results that at the same dose and same timing of phytosterol administration, the effects of PSE and YXD on reducing relevant levels are better than that of PS. However, multiple comparisons between the experimental groups with two different doses of phytosterols revealed that the reduction of TG levels in the groups given drugs with higher dose were more effective than in the groups given lower dose but the difference was not statistically signifi cant (P > 0.05). Thus, in term of theireffects on reduction of TG levels, there were no signifi cantly dose-dependent effects for PS, PSE and YXD. There was no signifi cant difference between the placebo group and the model group (P > 0.05), indicating that peanut oil that was used for dissolving PS and PSE has no signifi cant effects on this experimental index.
2.3 Effects of PS, PSE and YXD on antioxidant capacity in SD rats
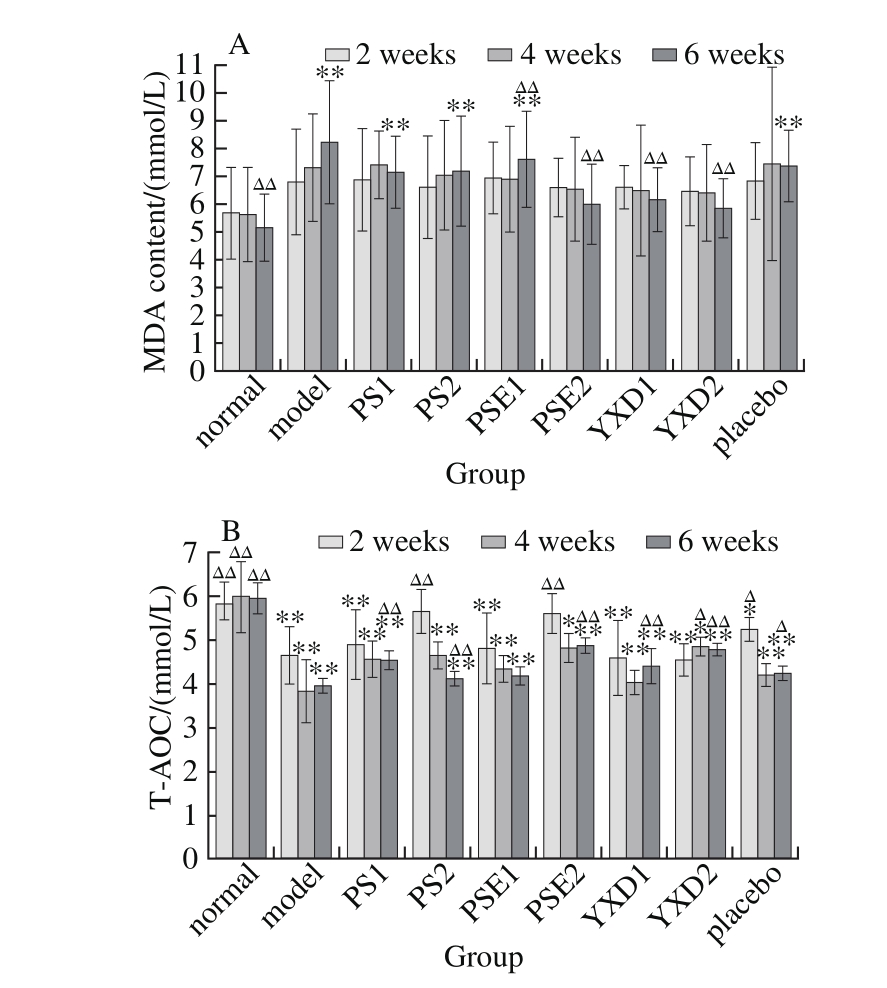
Fig. 2 Changes in MDA (A) and T-AOC (B) levels among different groups of rats
It can be seen from Fig. 2 that 2 weeks after phytosterol administration, there existed extremely signifi cant differences in the T-AOC of various experimental groups comparing with normal group (P < 0.01), indicating that administration with PS at higher doses and PSE can signifi cantly slow down the reduction in antioxidant capacity caused by the intake of high fats. Four and six weeks after phytosterol administration, there were significant differences (P < 0.05) or extremely significant differences (P < 0.01) in T-AOC between the normal group and various phytosterol-administrated groups, indicating that with extending of the feeding of rats with the high fat-diet, the T-AOC in the body is signifi cantly reduced, and that while administration SD rats with PS, PSE and YXD can slow down this reduction, their effects are not signifi cant. While for MDA content, 6 weeks after administration, there were no significant differences between normal groups and PSE2, YXD1 and YSD2 groups (P > 0.05). There were extremely significant differences in MDA content between the model group and PSE and between normal group and YXD groups (P < 0.01), indicating that administration of SD rats with PSE and YXD for 6 weeks cause a markedly signifi cant reduction of serum MDA levels.
Multiple comparisons between the experimental groups administrated with the same phytosterols but at two different doses revealed that administration of rats with these phytosterols at higher doses can cause more effective effects on slowing down the reduction of antioxidant capacity and that with extending of the administration timing, the effects were more significant. However, in term of their effects on MDA levels, there were no significant dose-response relationships for these phytosterols.
2.4 Measurements of the liver biochemical indexes of SD rats
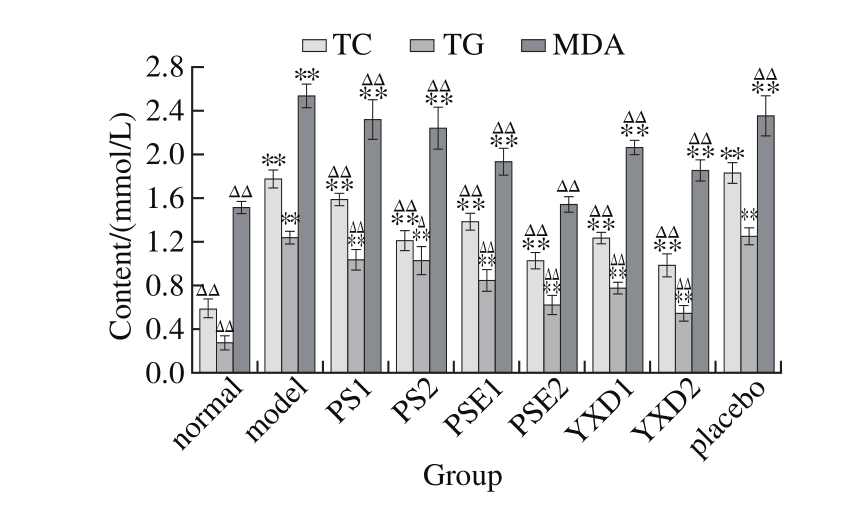
Fig. 3 Changes in liver TC, TG and MDA levels among different groups of rats
It can be seen from Fig. 3 that compared with those in the normal group and the model group, there existed extremely significant differences (P < 0.01) in the levels of TC, TG and MDA in the groups administrated with phytosterols but the levels of these indexes in the groups were still higher than that in the normal group. Compared with that in the normal group, there existed extremely signifi cant differences (P < 0.01) in the levels of TC, TG and MDA in all groups administrated with phytosterols except for the PSE2 group. Compared with that in the model group, there existed extremely significant differences (P < 0.0 1) in the levels of MDA in all the groups administrated with these phytosterols, indicating that administration of SD rats with these phytosterols for six weeks caused extremely signifi cant (P < 0.01) reduction of MDA levels, but there still existed very large differences as compared with that of the normal group, among which, the effects of PSE at high levels are more signifi cant.
Multiple comparisons between the experimental groups administrated with the same phytosterols but at two different doses revealed that there were markedly signifi cant differences in both TC and TG indexes between two groups(P < 0.01), Thus, in term of their effects on the levels of TC and TG, there are highly significant dose-response relationships in the groups administrated with these phytosterols. The groups with higher doses, the PSE2 and YXD2 groups, have better effects on reducing MDA levels. There existed significant differences in the effects of reducing the MDA between the groups with lower doses and the groups with higher doses. Thus, in term of their effects on MDA levels, there existed relatively significant dose-response relationships in various phytosterol administrated groups (experimental group).
2.5 The pathological sections in the various groups
The liver, heart and arterial arch pathological sections of SD rat were prepared according to the procedures described in section 1.3.4.3. Ten HE-stained pathological sections were taken from each treatment group and visualized with light microscope at 400× magnifi cation. Two fi elds of view were randomly selected for each section and observed. The liver, heart and arterial arch pathological severity were tested via nonparametric test, X2=32.889, P ≤ 0.01; X2=13.778, P ≥0.05; X2=20.889, P ≤ 0.01. The differences in pathological severit y among these groups were statistically significant. Thus, it is thought that the liver steatosis and membrane disorder in arterial arch were significantly improved in the SD rats of the experimental groups. The differences in the heart pathological severity among these groups were not statistically significant. The results were shown in Table 2, Fig. 4 to 6.

Fig. 4 Histological sections of livers of rats examined by HE staining (× 400)
Table 2 Comparison of pathological severity among different groups of rats (n= 10)
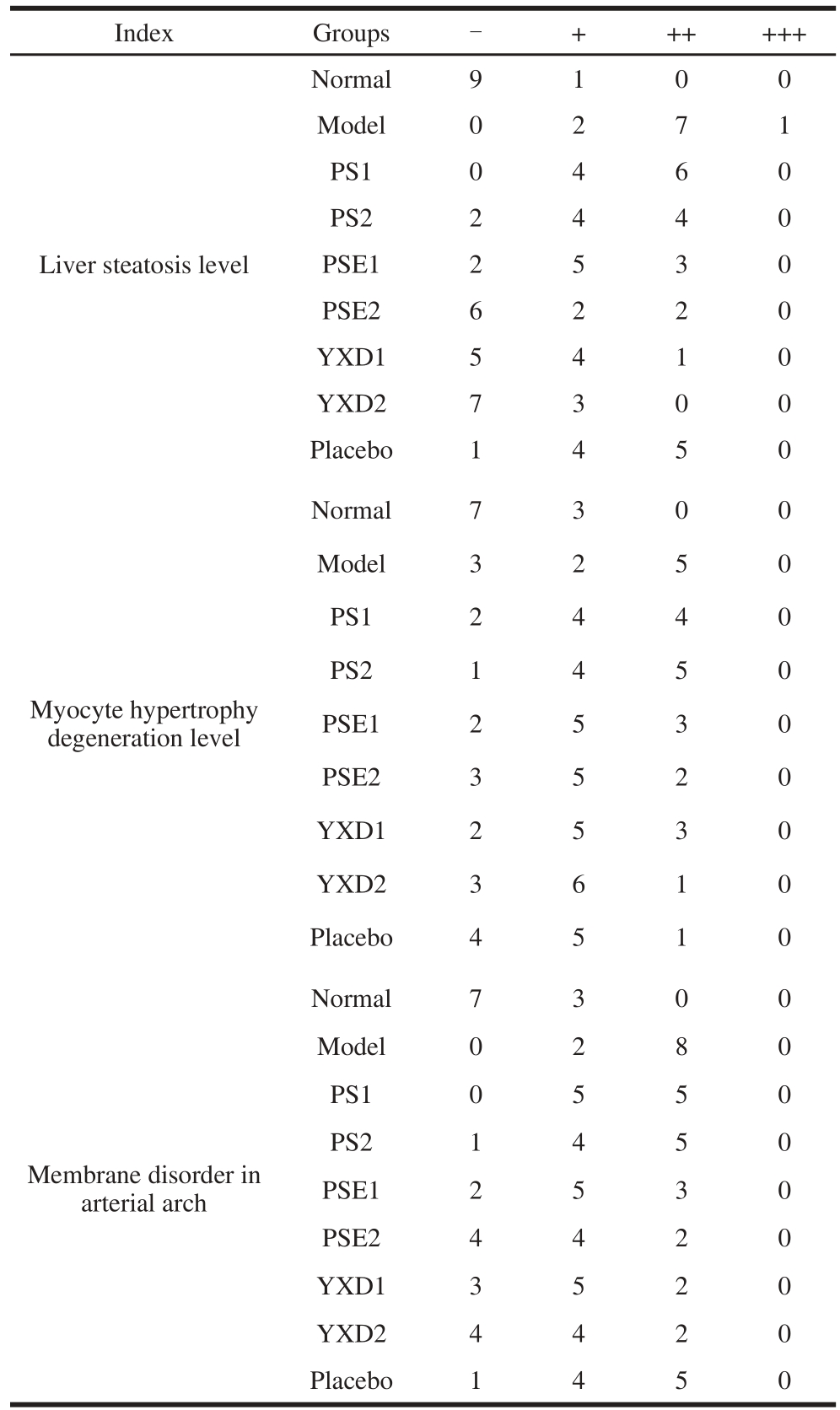
Note: -. pathological severity less than 30%; +. pathological severity 30%-50%; ++. pathological severity 50%-70%; +++. pathological severity more than 75%.
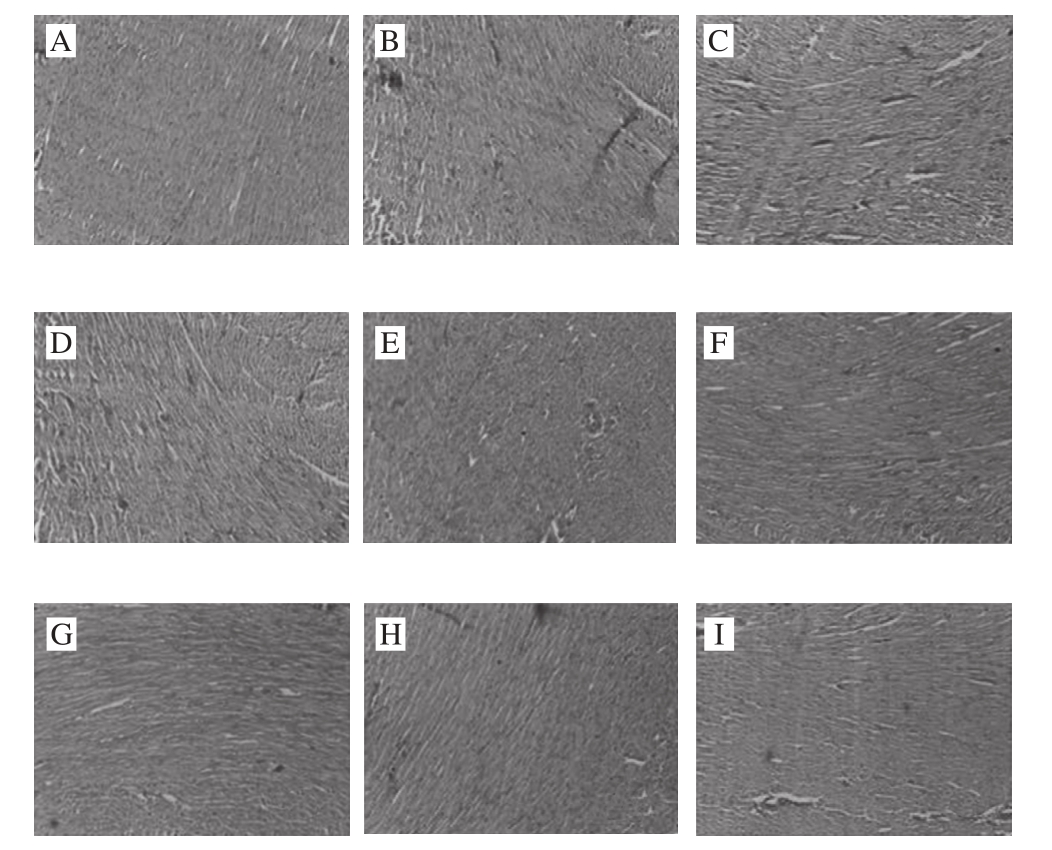
Fig. 5 Histological sections of hearts of rats examined by HE staining (× 400)
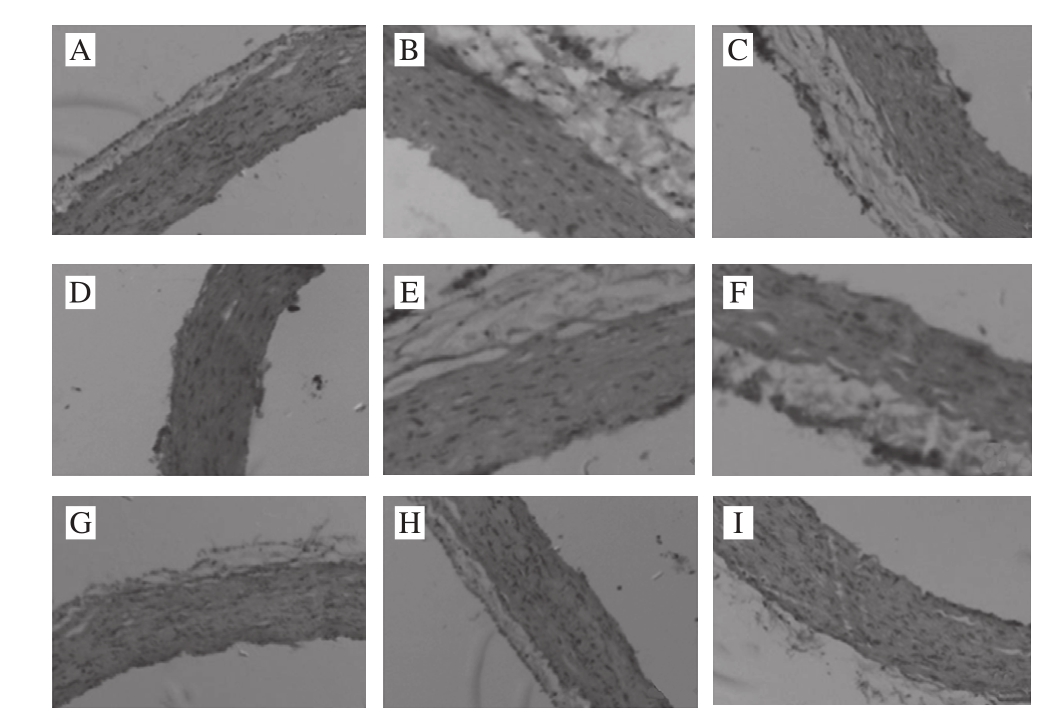
Fig. 6 Histological sections of arterial arch in rats examined by HE staining (× 400)
The steatosis was relatively suffused sand and there were more adipocytes infiltrated in the interspaces between hepatocytes. The cytoplasm of hepatocytes was loose and infiltrated with relatively heavy lipids. The nuclei were edged to one side and the cytoplasm was full of lipid droplets with varying sizes. In the PS1 group, the cytoplasm was stained red and the nuclei were dense and shrunken. Microvescular steatosis was seen in the hepatocytes but the severity was milder than that in the model group. In PS2 group, steatosis in hepatocytes was relatively suffused and was signifi cantly improved as compared to that in the model group. The severity of steatosis was reduced by about 20% as compared to that of the PS1 group, making the steatosis surrounding the central veins of liver milder. In the PSE1 group, hepatocytes were arranged in the radial symmetries surrounding the central vein. A part of hepatocytes with microvescular steatosis were still seen. Compared to that of the PS2 group, the severity of steatosis was relieved to certain extent. In the PSE2 group, compared to that of the normal group, the proportion of the hepatocytes without steatosis reached to 60% of that in the control group, indicating that different doses of the same phytochemical can significantly relieve the severity of steatosis. The severity of cellular disorganization was reduced by 30% compared to that in the group fed with high fat-diet, indicating that high fat diet had effects on the structure of aortae and lead to the alterations in cell morphology and disorganization of tissue arrangement whereas PSE and YXD at high doses can ameliorate vascular smooth muscle cell (VSMC) hypotrophy and showed certain preventive and ameliorative effects on the severity of tissue disorganization. However, there were no signifi cant effects on improving cardiac steatosis were seen in the groups administrated with PS, PSE and YXD. More importantly, examination of the HE-stained pathological sections of the YXD groups, which were the liquid dairy products in which PSE was added as the additive, revealed that steatosis was basically not observed in both YXD1 and YXD2 groups. Their capability of improving and relieving the steatosis was superior over those of the PS and PSE, indicating that formulation of dairy products with PSE as the additive can enhance the effects of PSE in ameliorating the intercellular steatosis in liver tissue. In the scientific aspect, these results demonstrate that the interactions among various substances can stimulate and enhance the tissue’s functional performance. From the perspective of maintaining human’s health, these results are beneficial for guiding the consumption and production of functional food products in a scientifi c way.
Investigators have studied the steroid substances for one hundred years from their discovery to the deep understanding of their physical and chemical properties. Earlier studies had mainly focused on the analysis and utilization of steroid molecules as the precursors for the synthesis of steroid hormones. In 1986, Heinemann et al.[19]firstly attempted to use PS substances to reduce the levels of total cholesterol in serum. Their study indicated that sitosterol was capable to capable to reduce the LDL-C levels in the patients with hyperlipidemia by 15%. Subsequently, a large number of clinical studies verified and ensured that PS conferred significant effects on changing the serum TC levels. Jones et al.[20]continuingly gave margarine equal to the intake of 1.84 g PS to patients with hyperlipidemia daily for three weeks and found that PS had signifi cant effects on reducing blood lipids and that the patient’s absorption of cholesterol was significantly reduced and the blood LDL-C level was significantly reduced whereas the levels of TG and HDL-C were not signifi cantly affected.
However, no studies were conducted to investigate PS, PSE and their related products together in the same animal model at the same time, and comprehensively analyzed their effects on the levels of several key CCD-related indexes in serum and liver. In this study, we took the dairy product, YXD, in which PSE was added as an additive, as an example, and we evaluated and verifi ed the effects of this dairy product on several functionally-related indexes. We also analyzed thepathological images of relevant organs from rats fed with this product. Our results confi rmed that the dairy product, YXD has clear heart-nourishing functions, especially in the aspects of preventing and reducing the risk of CCDs, protecting the arteries and maintaining the heart functions. Comparisons with other studies reported by a number of investigators in China and other countries have clearly demonstrated the values and signifi cance of this study. Therefore, we conducted an in-depth investigation on the effects of PS and its related product on the improvement and prevention of CCDs and the results obtained from this study would provide a scientific basis for the consumption and practice of the food-borne biologically active substances. In this study, we concluded that the intake of high fat-diet caused signifi cant pathological alterations in the histological and cellular morphology of liver and aorta whereas the intake of PSE and YXD at high doses can improve and ameliorate their pathological alterations to certain extents. They can effectively reduce the deposit of adipocytes in the liver tissue and the disorder and deformation of the outer membrane layers of aorta. These pathological alterations displayed are directly or indirectly correlated with the changing patterns of various indexes accessed in this study.
To date, it has been demonstrated that the abnormally high levels of cholesterol can induce dyslipidemia, hyperlipidemia and arteriosclerosis. Thus, to elucidate the mechanisms by which the plant-derived PS, PSE and their related products on reducing the risk of CCDs is of importance and scientifi c value. Based on the results obtained from this study, it is clear that the mechanisms by which PS and PSE reduce cholesterol are consistent with the action mechanisms proposed by Gylling[21]and Homma[22]et al., i.e. PS and PSE affect the cholesterol levels in the body mainly by affecting cholesterol transport. The results of this study indicate that intragastric administration of SD rats with PS, PSE and YXD can reduce LDL-C levels and increase HDL-C level. Because LDL is a lipoprotein responsible for transport of cholesterol, reducing its levels will retard the cholesterol transport process and thus, reduce the serum levels of cholesterol. HDL transports the extra hepatic cholesterol into liver where the excessive cholesterol is secreted and thus, regulates the cholesterol levels. Comparisons of the effects of PS, PSE and YXD at the same time points and at the same doses have revealed that the effects of YXD on reducing HDL-C and LDL-C levels are more signifi cant than those of PS and PSE. It can be inferred that some components in the dairy milk can generate synergistic effects on the cholesterolreducing indexes. These components may be casein in milk, from which some biologically active polypeptides can be generated via the intestinal proteases and these polypeptides are beneficial for the expression of LDL receptor in the cells[23], and thus, stimulates the absorption and degradation of LDL-C in serum. In addition, there is a unique medium that is beneficial for the absorption and transport of liposoluble substances in milk, which can stimulate and accelerate the transport and dissolution. Thus, PSE dissolved in milk can more effectively reduce the cholesterol levels. We observed that intragastric administration of SD rats with PS, PSE and YXD for six weeks significantly reduced the MDA levels in liver compared to that in the rats fed with high fat-diet and that the effects of PSE at high dose on reducing MDA levels were more signifi cant. Thus, MDA formed by the lipid peroxidation may act on LDL and affect LDL formation.
Our systemic assessment indicated that intragastric administration of SD rats with PS, PSE and YXD signifi cantly inhibited TC levels and increased HDL-C levels in SD rats. They also had certain effects on TG and T-AOC levels; Administration of YXD and PSE caused markedly signifi cant reduction of MDA content, confirming their significant antioxidant capacity. In addition, the changing trends in liverrelated indexes are consistent with conclusions drawn from the severity of steorease observed in the pathological images.
In this study, we found that PSE and the dairy product, YXD, had certain dose-response relationships for their effects on reducing the risk of CCDs. This study also confirmed that when PSE is added to dairy products as an additive, its effects on reducing the risk of CCDs, protecting arteries and maintaining heart smooth muscles are superior over those of the individual component. The results of this study have indicated that the mechanisms by which PS reduces cholesterol levels are consistent with the mechanisms proposed by Gylling et al.[21]. PS and PSE affect the cholesterol levels within the body mainly by affecting cholesterol transport. These results are of importance and have scientific value for evaluation and the in-depth investigation on the mechanisms by which PS and PSE and their related products in reducing cholesterol.
References:
[1] SHENG S. The effe ct of Qiongshao capsule on atherosclerosis rabbit blood lipid and infi ammatory factor[D]. Beijing: Beijing University of Chinese Medicine, 2013: 1-19.
[2] ZHANG L, CHEN Q S, YAN Y L, et al. Phytoste rols in the prevention and treatment of cardiovascular diseases[J]. Food Science, 2013, 34(23): 344-350. DOI:10.7506/spkx1002-6630-201323069.
[3] JI Guozhi, QIAN Wentao, CHEN Wei, et al. Study of depressing blood lipid on α-linolenic and plant sterol ester compound milk[J]. China Dairy Industry, 2012, 40(7): 35-36.
[4] CHEN Maobin, HUANG Qin, WU Moucheng. Compariso n of three phytosterol esters on preventing diet-induced hyperlipidemia in mice[J]. Journal of the Chinese Cereals and Oils Association, 2005, 20(2): 80-82.
[5] AI L Y, XU J, LUO F J, et al. A intervention study on cholesterollowering effect of phytosterol ester[J]. Capital Journal of Public Health, 2012, 6(1): 18-20.
[6] GUO Yubao, QIU Aiyong. Study on blood-fat-lowering effect of phytosterol esters[J]. China Oils and Fats, 2003, 28(9): 49-51.
[7] THORNTON S J, WONG I T, NEUMANN R, et al. Dietary supplementation with phytosterol and ascorbic acid reduces body mass accumulation and alters food transit time in a diet-induced obesity mouse model[J]. Lipids in Health and Disease, 2011, 10(1): 1-14.
[8] TIKKANEN M J, HÖGSTRÖM P, TUOMILEHTO J, et al. Effect of a diet based on low-fat foods enriched with nonesterified plant sterols and mineral nutrients on serum cholesterol[J]. American Journal of Cardiology, 2001, 88(10): 1157-1162. DOI:10.1016/S0002-9149(01)02053-7.
[9] HALLIKAINEN M A, SARKKINEN E S, UUSTITUPA M I. Plantstanol esters affect serum cholesterol concentrations of hyper cholesterolemic men and women in a dose-dependent manner[J]. The Journal of Nutrition, 2000, 130(4): 767-776.
[10] REMAUD G, DALAN E, PIGUET C, et al. Effects of non-esterifi ed sterols in a liquid emulsion on cholesterol absorption and synthesis in hypercholesterolemic men[J]. European Journal of Nutrition, 2002, 41(2): 54-60. DOI:10.1007/s003940200008.
[11] BLAIR S N, CAPUZZI D M, GOTTLIEB S O, et al. Incremental reduction of serum total cholesterol and low-density lipoprotein cholesterol with the addition of plant stanol ester-containing spread to statin therapy[J]. American Journal of Cardiology, 2000, 86(1): 46-52. DOI:10.1016/S0002-9149(00)00976-0.
[12] NYUGEN T T, DALE L C, BERGMANN K, et al. Cholesterollowering effect of stanol esterin a US population of mildly hyper-cholesterolemic men and women a randomized controlled trial[J]. Mayo Clinic Proceedings, 1999, 74(12): 1198-1206. DOI:10.4065/74.12.1198.
[13] ZHANG Q, WU P, QU D, et al. Preventive effects of phytosterol ester on high fat diet-induced non-alcoholic fatty liver disease in rats[J]. World Chinese Journal of Digestology, 2014, 22(34): 5242-5248. DOI:10.11569/wcjd.v22.i34.5242.
[14] AMIOT M J, KNOL D, CARDINAULT N, et al. Phytosterol ester processing in the small intestine: impact on cholesterol availability for absorption and chylomicron cholesterol incorporation in healthy humans[J]. Journal of Lipid Research, 2011, 52(6): 1256-1264. DOI:10.1194/jlr.M013730.
[15] WANG Y, LIU B C, REN Y H, et al. Lipid-lowering effect of phytosterol ester in hyperlipidemic rats[J]. Food Science, 2011, 32(17): 326-329.
[16] SANTAS J, CODONY R, RAFECAS M. Phytosterol s: beneficial effects[M]. Berlin Heid elberg: Springer, 2013: 3437-3464. DOI:10.1007/978-3-642-22144-6_149.
[17] MOREAU R A, WHITAKER B D, HICKS K B. Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses[J]. Progress in Lipid Research, 2002, 41(6): 457-500.
[18] PAN Lin. E xperimenta l pathology map technology[M]. Beijing: Science Press, 2012: 60-80. DOI:10.1016/S0163-7827(02)00006-1.
[19] HEINEMANN T, LEISS O, BERGMANN K V. Effect of low-dose sitostanol on serum cholesterol in patients with hypercholesterolemia[J]. Atherosclerosis, 1986, 61(3): 219-223. DOI:10.1016/0021-9150(86)90141-3.
[20] JONES P J, RAEINI-SARJAZ M, NTANIOS F Y, et al. Modulation of plasma lipid levels and cholesterol kinetics by phytosterol versus phytostanol esters[J]. Journal of Lipid Research, 2000, 41(5): 697-705.
[21] GYLLING H, PUSKA P, VARTIAINR E, et al. Serum sterols during stanolester feeding in a mildly hypercholesterolemic population[J]. Journal of Lipid Research, 1999, 40(4): 593-600.
[22] HOMMA Y, IKEDA I, ISHIKAWA T, et al. Decrease in plasma low density lipoprotein cholesterol, apolipoprotein B, cholesterylester transfer protein, and oxidized low-density lipoprotein by plant stanol ester containing spread: a randomized, placebo controlled trial[J]. Nutrition, 2003, 19(4): 369-374.
[23] PANG G C, CHEN Q S, HU Z H, et al. Bioactive peptides: absorption, utilization and functionality[J]. Food Science, 2013, 34(9): 375-391.
植物甾醇及添加植物甾醇乳制品降低心脑血管疾病风险的功能性评价
明 珠,陈庆森*,张 蕾,闫亚丽,赵 培
(天津商业大学生物技术与食品科学学院,天津市食品生物技术重点实验室,天津 300134)
摘 要:本研究对植物甾醇(phytosterol,PS)、植物甾醇酯(phytosterol ester,PSE)及其相关乳制品进行降低心血管疾病风险方面的功能性评价,并为指导植物性化学物质和养心乳制品(Yangxin dairy,YXD)的实际使用提供科学依据。SD大鼠被分为9 组,每组10 只。正常对照组、模型组大鼠分别喂养正常饲料和高脂饲料,灌胃0.9%生理盐水,PS、PSE、YXD和安慰剂组高脂喂养的同时连续灌胃PS(23.6、47.2 mg/mL)、PSE(23.6、47.2 mg/mL)、YXD(30、60 g/250 mL)和花生油 4 周。4 周后处死所有大鼠取样本进行检测。结果发现,大鼠总胆固醇含量明显降低,高密度脂蛋白胆固醇含量呈剂量依赖性增加。这些干预方式会对降低甘油三酸酯的水平、总抗氧化能力和丙二醛含量有一定影响,但效果不稳定。在肝脏和病理切片的相应指标测定结果中也有相同的趋势。PSE和YXD能有效地降低心脑血管疾病风险。此外,本研究证实YXD在降低心血管疾病风险、保护肝脏和血管的效果方面较突出。
关键词:植物甾醇;植物甾醇酯;心脑血管疾病;降胆固醇
中图分类号:TS218
文献标志码:A
文章编号:1002-6630(2017)17-0174-10
收稿日期:2016-06-20
基金项目:国家自然科学基金面上项目(31071522);天津市教委面上项目(20120603)
作者简介:明珠(1992—),女,硕士研究生,研究方向为发酵生物技术、功能成分与肠道健康的关系。E-mail:ming_zhu999@126.com
DOI:10.7506/spkx1002-6630-201717029
*通信作者:陈庆森(1957—),男,教授,硕士,研究方向为发酵生物技术、功能成分与肠道健康的关系。E-mail:chqsen@tjcu.edu.cn