天然产物与microRNA调控代谢综合征的研究进展
郭 钦1,白 洁1,何宇轩1,唐川惠1,操庆国1,2,董 英1
(1.江苏大学食品与生物工程学院,江苏 镇江 212013;2.江苏农林职业技术学院生物工程系,江苏 句容 212000)
摘 要:代谢综合征已成为全球性的健康问题。microRNA(miRNA)是一类能够调节基因表达的短单链內源非编码RNA(约22 nt),在转录后水平调节基因表达。miRNA在代谢综合征中发挥重要作用,药食同源的天然产物可以通过调控miRNA的活性和功能,改善糖脂代谢紊乱。本文主要综述miRNA在糖尿病、肥胖 等代谢综合征的致病机制中的研究进展,深入分析了药食同源的天然产物对miRNA的调控机制,可为发掘miRNA在代谢综合征预防、诊断、治疗中的潜在价值提供理论依据。
关键词:代谢综合征;microRNA;天然产物
代谢综合征(metabolic syndrome,MetS)是一组以肥胖、高血糖或糖调节异常、血脂异常和高血压等聚集发病为特征的,严重影响人类健康的临床征候群。随着人们生活方式的改变,以营养性肥胖和Ⅱ型糖尿病为代表的代谢综合征已成为影响人类健康最主要的非传染性慢性疾病之一[1]。MetS的发病机制十分复杂,涉及与遗传和环境因素相互作用有关的诸多因素,美国心脏学会、心肺血液研究所、糖尿病学会发表的临床共识中,将MetS的病因归纳为以下3 个方面:肥胖、脂肪组织功能障碍、胰岛素抵抗[2]。2005年4月,国际糖尿病联盟(the international diabetes federation,IDF)在MetS新定义中提出中心性肥胖与胰岛素抵抗是公认的重要致病因素,强调中心性肥胖为MetS核心组分[3]。
microRNA( miRNA)是一类能够调节基因表达的短单链内源非编码RNA(约22 nt),通过与靶基因3’非翻译区互补配对结合,降解或者抑制靶基因mRNA的翻译,从而在转录后水平对基因的表达进行调控。已有大量研究表明,miRNA在一系列生物过程如增殖、分化、凋亡和发展中具有不同的调控作用[4],它的表达异常引起相应调控网络紊乱是疾病发生的重要原因[5]。
饮食营养是影响人类健康和疾病的重要可调控因素,通过食品或药食同源的食品调节人体糖脂代谢过程,开发天然、安全有效的食源性调脂降糖产品一直是食品营养学研究的热点之一,已发现越橘、蓝莓、桑树叶、香菇、苦丁茶、马齿苋等250多种天然产物及其提取物具有降脂、降糖、保护β-细胞和改善胰岛素抵抗等功能且没有毒副作用[6]。目前,大量流行病学及临床研究发现,天然产物营养可以调控miRNA表达,缓解疾病症状[7]。因此,深入研究miRNA与代谢综合征的调控关系,探索天然营养调控miRNA缓解代谢疾病的机制,将对代谢综合征的早期防治和治疗有重大意义,现对miRNA与糖尿病、肥胖、胰岛素抵抗、脂肪组织等的关系进行了综述并分析其分子标记物作用,以便人们在此基础上进一步发掘miRNA在代谢综合征预防、诊断、治疗中的潜在价值。
1 miRNA与糖尿病
多个miRNA与糖尿病的发生发展密切相关(表1)。研究表明,30/384 个和29/283 个miRNA在胰岛和胰岛素靶组织(肝脏、脂肪组织和肌肉组织)中有差异表达,其中miR-222和miR-27a在脂肪组织中显著上调,miR-195和miR-103在肝脏组织中上调,miR-10b在肌肉组织中下调[8]。许多miRNA具有显著的组织特异性,miR-375具有胰岛特异性[9],miR-103/107、miR-335等具有肝脏特异性[10],miR-143具有脂肪组织特异性[11],miR-133a则具有心脏特异性[12]。也有一些miRNA在多个组织中普遍差异性表达,在不同组织中行使不同功能从而调控糖尿病及相关并发症。miR-29a在脂肪组织中上调导致血糖增加在心肌组织中表达下调会导致糖尿病心肌损伤;miR-21在Ⅰ型糖尿病周边血细胞中显著下调,靶向下调同源性磷酸酶-张力蛋白(phosphatase and tensin homolog,PTEN),从而增加核因子(nuclear factor kappa B,NF-κB)活性,正向调控炎症反应[13],同时,也在Ⅱ型糖尿病肾病小鼠肾脏中上调,下调Smad7水平,促进糖尿病肾损伤[14]。
miRNA可能通过直接调控胰岛素分泌或者胰岛素靶组织的分化和脂代谢,诱导胰岛素抵抗,从而引发糖尿病。目前已有大量研究通过动物模型或者细胞模型,确定了胰岛素抵抗相关的miRNA,并分析了miRNA与胰岛素抵抗相关临床症状之间的关系,深入揭示了miRNA在糖尿病中的重要调控作用(表2),包括miR-375、miR-221/ miR-124a、miR-9、miR-29a/b/c、miR-107/103和miR-93。miR-375在胰腺发育的过程中逐渐增加,是胰β细胞形成的本质因素[21]。在成熟的胰岛中miR-375可以抑制丙酮酸脱氢酶激酶1(pyruvate dehydrogenase kinase 1,PDK1)[22]和重组胰岛素样生长因子(myotrophin, Mtpn)[23]的表达,调控低葡萄糖刺激引起的胰岛素分泌。miR-221可以调控肿瘤坏死因子(tumor necrosis factor,TNFα)[24]和脂联素受体1(adiponectin receptor 1,ADIPOR1)[25]从而调控胰岛素分泌。脂代谢在维持葡萄糖稳态中也具有重要的作用,miR-143直接作用于胰岛素反应性葡萄糖转运体4(insulin-responsive glucose transporter 4,GLUT-4)和过氧化物酶体增殖物激活受体γ(peroxisome proliferator activated receptor γ,PPARγ),控制胆固醇和脂肪酸生成;miR-145直接下调TNF-α,调节脂质代谢。由于以上这些实验都是以大鼠或小鼠为模型或者以细胞为模型,其结果均未曾在人体中得到证实,因此这些miRNA距离临床应用仍需要进一步深入研究。
表1 miRNA在糖尿病中的表达谱
Table1 Expression prof i le of miRNA in diabetes

文献来源研究方法组织上调基因下调基因功能Herrera等[8]基因芯片/ RT-PCR脂肪组织miR-222/miR-27a,miR-29a脂肪组织中差异肝脏miR-103/miR-195miR-126肌肉miR-10b性表达的miR-22 2/ miR-27a/miR-29a增加血糖水平Esguerra等[15]基因芯片/ RT-PCR胰岛miR-130a/miR-132/ miR-212/miR-335/ miR-142-3p/miR-124/ miR-376a/miR-433/ miR-142-5p/miR-409-3p/miR-375 miR-708调控血糖刺激引起的胰岛素分泌Arner等[16]基因芯片/ RT-PCR皮下白色脂肪miR-222/ miR-342-3p let-7a/let-7d/let-7i/miR-16/miR-26a/miR-30c/miR-92a/miR-126/miR-139-5p/miR-143/ miR-145/miR-151-5p/miR-193a-5p/ miR-193b/miR-197/miR-484-5p/ miR-378/ miR-652 let-7d/miR-26a/ miR-30c/ miR-145、miR-193和miR-652调控脂质代谢;miR- 143/miR-145 miR-378影响脂肪细胞分化Capobianco等[17]基因芯片/ RT-PCR miR-144和miR-520e调节葡萄糖代谢Diawara等[18]候选基因法脂肪组织miR-125a未确定Chen Ling等[19]候选基因法脂肪组织miR-146b未确定内脏白色脂肪miR-141和miR-520刁雪红等[20]基因芯片/ RT-PCR心肌组织miR-195、miR-199a-3p、miR-700、miR-142-3p、miR-24、miR-2l、miR-221、miR-499-3P、miR-208a、miR-705 miR-29a、miR-l、miR-37 3、miR-143、miR-20a、miR-220未确定
随着miRNA参与糖尿病致病机制研究的不断深入,大量研究揭示了miRNA在血液、血浆以及血细胞中的功能,从理论上证实了某些循环miRNA也许可以作为检测、判断糖尿病的生物标记物。比如根据血清中hsa-miR-1274a、hsa-miR-1274b和hsa-let-7f可以判定Ⅰ型糖尿病(type 1 diabetes,T1D);hsa-miR-222、hsa-miR-30e和hsamiR-140-3p可以判定Ⅱ型糖尿病(type 2 diabetes,T2D);hsa-miR-181a和hsa-miR-1268可以判定妊娠糖尿病(gestational diabetes mellitus,GDM)[43]。但是这些研究结果存在临床上的不一致性。例如,Kong Lei等[44]以新确诊的Ⅱ型糖尿病患者(newly diagnosed T2D,n-T2D)、糖耐量受损的早期糖尿病患者(impaired glucose tolerance/ impaired fasting glucose,IGT/IFG)和Ⅱ型糖尿病易感患者(T2D-susceptible individuals,s-NGT)为对象,对比了他们血清中7 个糖尿病密切相关的miRNA的差异性表达谱,结果只有miR-34a持续发生显著性变化;Zhang Tao等[27]以正常人群、糖尿病易感人群以及糖尿病患者为对象,对比了miR-29b、miR-28-3p、miR-15a、miR-223和miR-126的差异性表达,结果只有miR-126在糖尿病易感人群和糖尿病患者中显著下调。有研究发现[45],miR-144具有显著的种族差异性,它只在瑞典糖尿病患者体内高表达,而在伊拉克患者中却没有显著差异;靶基因APOL6结合区的多样性会影响其与miR-143和miR-24的结合率,从而影响代谢综合征的易感性[46]。由于miRNA的表达受种族、性别、地区、环境、饮食和个体的差异影响较大,目前多项研究的结果存在差别甚至矛盾,因此需要全面分析miRNA在糖尿病中的作用机制并结合基因组学、转录组学和有效的临床实验,才能实现循环miRNA作为糖尿病的生物标记物和治疗靶标的作用。
表2 miRNA在糖尿病中调控作用
Table2 Regulatory role of miRNA in diabetes

文献miRNA靶基因作用Meng Shu等[26]miR-126Spred-1造成糖尿病中内皮祖细胞功能紊乱Zhang Tao等[27]miR-126未确定导致糖尿病的发生Poy等[23]miR-375Mtpn调控葡萄糖引起的胰岛素分泌El Ouaam ari等[22]miR-375PDK-1调控胰岛素合成,诱导胰岛素抵抗Meerson等[24]miR-221TNF调控胰岛素分泌Chou等[25]miR-221ADIPOR1 Yaniv等[28]miR-221ADIPOR1提高脂联素的有效作用,改善胰岛素抵抗Baroukh等[29]miR-124aFoxA2调控胰岛分化和胰岛素分泌,维持血糖平衡Lovis等[30]miR-124aRab27A调控胰岛素分泌Valérie等[31]miR-9Onecut2调控血糖刺激的胰岛素分泌miR-9Granuphilin下调胰岛素分泌He Aibin等[32]miR-29a/b/cCav2调控胰岛素分泌,与胰岛素抵抗相关Mirko等[33]miR-107/103caveolin1降低胰岛素敏感性Chen Lin等[34]miR-143GLUT-4和PPARy控制胆固醇和脂肪酸生成Lorente-Cebrián等[35]miR-145TNF-α调节脂质代谢史春梅等[36]miR-26b未确定改善人脂肪细胞炎症状态及改善胰岛素抵抗Gao Song等[12]miR-133aIGF-1R调控平滑肌细胞增殖Balasubramanyam等[37]miR-146a未确定调控胰岛素分泌和临床炎症Salas-Péreza等[38]miR-21和miR-93未确定调控T细胞功能,导致临床炎症Zhong等[39]miR-21Smad7调控糖尿病肾损伤Feng Biao等[40]miR-1ET-1导致各组织功能紊乱,引起糖尿病并发症Kato等[41]miR-192SIP1调控肾功能,导致糖尿病肾病Chen Shali等[42]miR-133aERK1/2和SMAD2导致糖尿病引起的心肌纤维化Esguerra等[15]miR-335Stxbp1调控血糖刺激引起的胰岛素分泌
2 miRNA与肥胖
肥胖是心血管疾病的危险因素,与代谢综合征密切相关。肥胖症主要由遗传、环境等多种因素相互作用引起,但是肥胖的致病机制仍然不清楚。大量研究对比了miRNA在肥胖症和正常人中的表达谱,在非糖尿病肥胖小鼠的皮下脂肪中,42/1 458 个miRNA发生了差异性表达[47];基因芯片技术确证了几个在糖尿病小鼠体内正常而在肥胖和糖尿病肥胖小鼠体内表达紊乱的miRNA[16-17,48-50],以及一些只在肥胖中表达紊乱的miRNA(表3),某些miRNA的调控作用也得到了初步确定(表4)。比如miR-34a和miR-146a等在肥胖小鼠胰岛组织中表达下调,调控脂代谢和胰岛素抵抗,miR-103/107、miR-335等在肥胖糖尿病小鼠的肝脏组织中表达上调,调控肝脏代谢和脂肪细胞分化及脂质代谢;miR-519d在肥胖和糖尿病人体的皮下脂肪组织中上调,调控脂肪酸生成。
表3 miRNA在肥胖中的表达谱
Table3 Expression prof i le of miRNA in obesity

文献组织上调下调在肥胖中的作用Heneghan等[48]内脏脂肪无miR-17-5p和miR-132调控免疫系统Martinelli等[47]皮下脂肪miR-519dmiR-150和miR-659未确定Ortega等[50]皮下脂肪miR-99a、miR-199a-5p、miR-125b、miR-221和miR-1229 miR-130b、miR-139-5p、miR-185 and miR-484未确定Keller等[49]皮下脂肪miR-21miR-143未确定Meerson等[24]皮下脂肪miR-221miR-193a-3p和miR-193b-5p未确定Capobianco等[17]内脏脂肪无miR-141和miR-520miR-141和miR-520可以调控糖脂代谢
表4 miRNA在肥胖中的调控作用
Table4 Regulatory role of miRNA in obesity
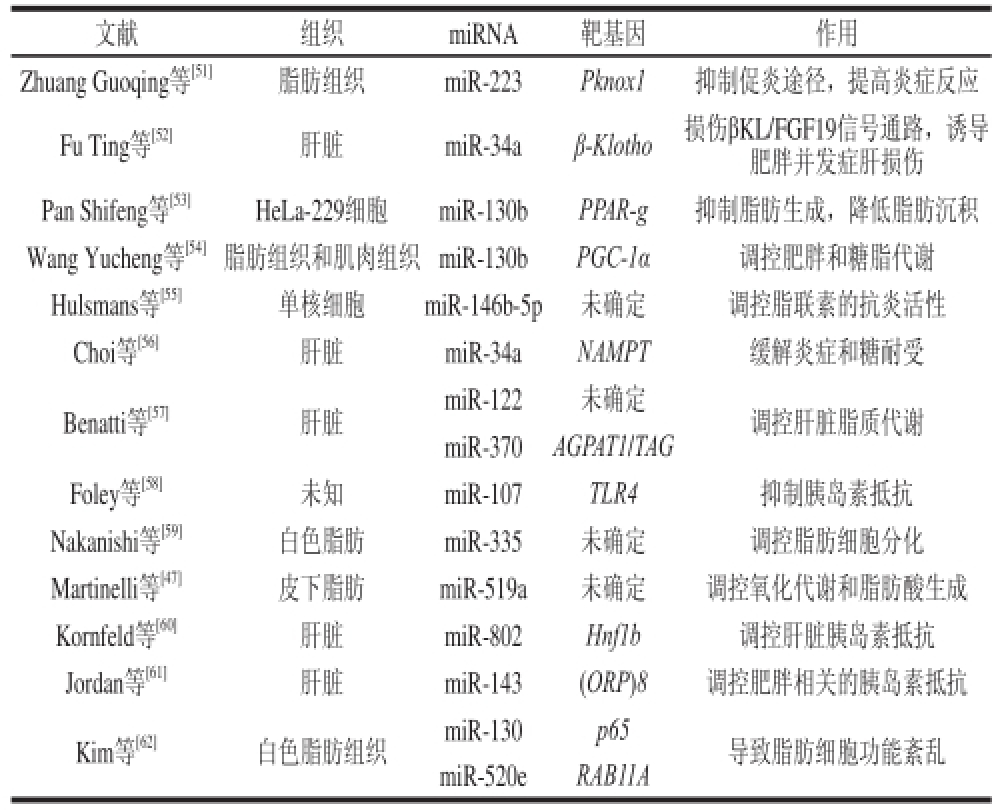
文献组织miRNA靶基因作用Zhuang Guoqing等[51]脂肪组织miR-223Pknox1抑制促炎途径,提高炎症反应Fu Ting等[52]肝脏miR-34aβ-Klotho损伤βKL/FGF19信号通路,诱导肥胖并发症肝损伤Pan Shifeng等[53]HeLa-229细胞miR-130bPPAR-g抑制脂肪生成,降低脂肪沉积Wang Yucheng等[54]脂肪组织和肌肉组织miR-130bPGC-1α调控肥胖和糖脂代谢Hulsmans等[55]单核细胞miR-146b-5p未确定调控脂联素的抗炎活性Choi等[56]肝脏miR-34aNAMPT缓解炎症和糖耐受Benatti等[57]肝脏miR-122未确定调控肝脏脂质代谢miR-370AGPAT1/TAG Foley等[58]未知miR-107TLR4抑制胰岛素抵抗Nakanishi等[59]白色脂肪miR-335未确定调控脂肪细胞分化Martinelli等[47]皮下脂肪miR-519a未确定调控氧化代谢和脂肪酸生成Kornfeld等[60]肝脏miR-802Hnf1b调控肝脏胰岛素抵抗Jordan等[61]肝脏miR-143(ORP)8调控肥胖相关的胰岛素抵抗Kim等[62]白色脂肪组织miR-130p65导致脂肪细胞功能紊乱miR-520eRAB11A
大量的临床实验已经证实了miR-142-3p、miR-140-5p、miR-15a、miR-520c-3p和miR-423-5p可以作为病态肥胖的风险评估和分类的标记物[62]。血清中的miR-27a与皮下脂肪和身体质量指数(body mass index,BMI)相关,血清miR-122仅与内脏脂肪相关[63];这些结论为miRNA的生物标记物作用提供了理论依据。然而,循环miRNA与肥胖等代谢综合征的关系具有一定的性别依赖性[64]和年龄依赖性等[65]。因此,在对miRNA进行深入研究时,也需要考虑个体miRNA的差异性。
此外,miRNA在病态肥胖中差异性表达会随着体质量的降低而发生改变。当miRNA在机体内缺失时,肥胖小鼠的一些相关代谢问题会得到扭转。miR-34a在肥胖动物的肝脏、脂肪肝疾病患者中处于高水平,当采用反义RNA技术去中和阻断肥胖小鼠中的miR-34a时,高脂肪饮食导致肥胖小鼠的肝脏脂肪降低、血液中的葡萄糖水平得到改善[51];miR-378和miR-378*缺陷小鼠也能够快速地将细胞食物转化为能量,抵抗肥胖[66];miR-103/107在健康小鼠体内过表达会引起高血糖症状[29];这些研究提示了靶向特异性miRNA的治疗方法可能有助于抑制肥胖症。
3 其他代谢疾病
糖尿病肾病(diabetic nephropathy,DN)是糖尿病并发症中最为严重的之一,已成为导致终末期肾衰竭的重要原因,也是导致糖尿病患者死亡的主要原因,严重威胁人们的健康。在糖尿病肾病小鼠的肾脏中,66 个miRNA发生了显著的差异性表达[67]。miR-192在糖尿病肾病中有重要作用,其在糖尿病小鼠肾脏中表达上调,肾小球系膜细胞中的转化生长因子(transforming growth factor beta 1,TGF-β1)可以上调miR-192,使其与col1a2基因的E-box区结合,通过抑制E-Box抑制因子(zinc fi nger E-box binding homeobox 1/2,ZEB1/2)的表达,增加体内的胶原蛋白沉积,另外miR-192也可以通过TGF-b1/Smad信号通路,调控纤维化肾病发病程度[37]。miR-21也在糖尿病肾病小鼠体内表达下调,抑制PTEN的表达,增加尿蛋白[68]。
动脉硬化是代谢综合征的另一种体现,血管内皮细胞损伤是动脉粥样硬化发病的关键初始环节。研究发现,多种miRNA在内皮细胞中呈特异性表达,如miR-126、miR-22l、miR-222、miR-130a、let-7家族、miR-2l和miR-27b在血管内皮细胞中呈高表达。miR-145[69]在平滑肌细胞中的表达有选择性,通过直接调控锌指转录因子5、锌指转录因子4和钙调节蛋白激酶Ⅱδ来调节平滑肌细胞功能。miR-503[70]直接作用于细胞周期蛋白(cyclin E1,CCNE1)和细胞分裂周期25家族蛋白(cell division cycle 25A,cdc25A),调控内皮细胞功能。但是miRNA在糖尿病引起的动脉硬化中的调控作用仍需要进一步探讨。
糖尿病心肌症主要表现为心肌肥大、心脏功能紊乱,最终导致心脏衰竭。研究发现,多种miRNA在糖尿病患者心脏中呈特异性表达,如miR-29、miR-320、miR-21、miR-30和miR-133。miR-133[71]具有心脏特异性,能够调控肌细胞生成,它在糖尿病患者心脏中具有双重作用,糖尿病会导致心脏中血清效应因子增加,引起miR-133表达上调,从而抑制心肌肥大,而降低miR-133的表达又会导致心肌肥大,因此在血清效应因子、miR-133以及心肌肥大之间存在着反馈调节网络,如何平衡miR-133的表达时治疗糖尿病心肌症的关键。
4 天然产物与miRNA
miRNA可以通过多种膜运输小泡、蛋白、脂蛋白以及核糖核酸复合物等机制在细胞间流通[72],实现细胞间的信息交流。除了机体本身的miRNA外,来自食物的外源miRNA可能也对机体存在一定的调控作用。2012年,Zhang Lin等[73]发现,大米中的特异性miR-168a可以进入动物血清以及健康人血清中,并可以调控小鼠肝脏中的低密度脂蛋白-1,揭示了食物中的遗传物质可以通过大肠进入血液;除此之外,血清中的循环miRNA也可能来源于一些外源物种,如细菌和真菌[74]。有研究发现,母乳中也有大量的免疫相关miRNA,可以抑制胃中的低酸水平[75],miR-375通常表达在胰腺,小肠和乳腺中,近期,研究员用表达miR-375的野生型母鼠的乳汁哺育敲除幼鼠,对敲除幼鼠胃部的乳汁进行检测,发现了高浓度的miR-375,而在敲除幼鼠的身体其他部位只能检测到微量的miR-375,并且这些微量microRNA的浓度不足以发挥基因调节功能。除此之外,在血液和肝脏中几乎不存在miR-375[76],因此,推测母乳中的miRNA或许能够通过靶向作用于婴儿的mRNA。然而这些推测都未得到确证,外源miRNA的稳定性及其调控机制仍需进一步深入研究。
大量临床和流行病学研究表明,饮食是影响健康与疾病的可调控因素,很多药食同源的天然产物能够调控miRNA的表达,改善其造成的糖尿病小鼠的一些病理特征(表5)。丹皮酚上调miR-126的表达,抑制PI3K/Akt/NF-κB信号通路的转导[103];丹酚酸B可以下调miR-106b的表达,抑制TGF-βRⅡ,调控TGF-β1/Smads信号通路[107];Omega-3多不饱和脂肪酸能够降低miR-146b的表达,上调Smad4,调控TGF-β信号通路,发挥其抑制炎症的作用[104];体内敲除miR-21可以提高肾 功能,减轻糖尿病引起的肾脏损伤,抑制肾脏纤维化和肾脏炎症的发生[106];本课题组前期实验结果中也发现,苦瓜超微冻干粉可以改善肥胖SD大鼠的病理状态,并改变其肝脏中miR-34a-5p、miR224-3p、miR-1-3p、miR-221-5p差异表达,从而改善肥胖SD大鼠的病理状态,其中miR-34a-5p在肥胖组对正常组和苦瓜组对肥胖组中分别表现为上调和下调;miR224-3p、1-3p、miR221-5p在肥胖组对正常组和苦瓜组对肥胖组中分别表现为下调和上调,由此推测苦瓜可以改善这4 个miRNA的表达,使其趋于正常,从而改善肥胖。
表5 天然产物调控miRNA
Table5 miRNA regulation by natural products
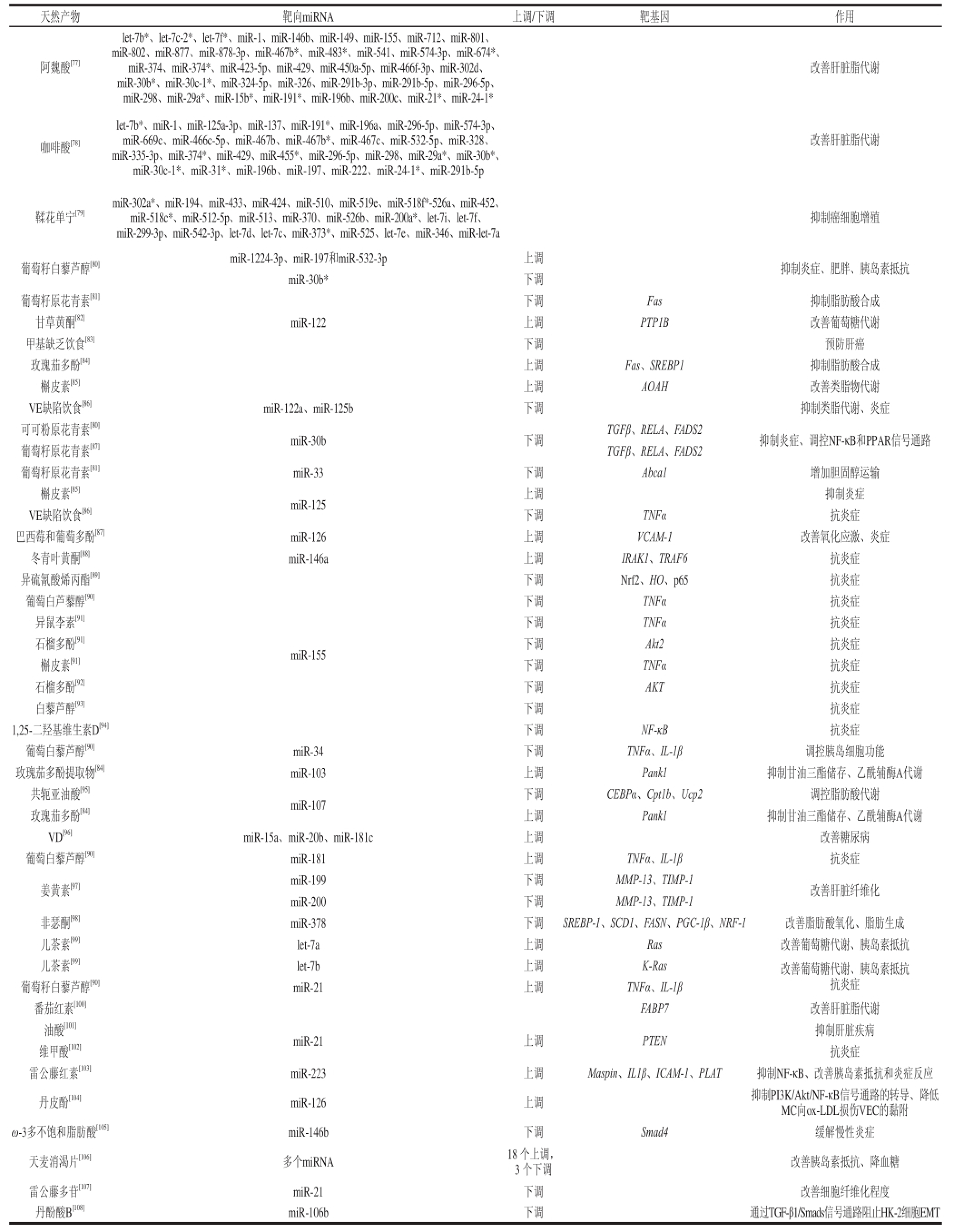
阿魏酸[77]let-7b*、let-7c-2*、let-7f*、miR-1、miR-146b、miR-149、miR-155、miR-712、miR-801、miR-802、miR-877、miR-878-3p、miR-467b*、miR-483*、miR-541、miR-574-3p、miR-674*、miR-374、miR-374*、miR-423-5p、miR-429、miR-450a-5p、miR-466f-3p、miR-302d、miR-30b*、miR-30c-1*、miR-324-5p、miR-326、miR-291b-3p、miR-291b-5p、miR-296-5p、miR-298、miR-29a*、miR-15b*、miR-191*、miR-196b、miR-200c、miR-21*、miR-24-1*改善肝脏脂代谢咖啡酸[78]let-7b*、miR-1、miR-125a-3p、miR-137、miR-191*、miR-196a、miR-296-5p、miR-574-3p、miR-669c、miR-466c-5p、miR-467b、miR-467b*、miR-467c、miR-532-5p、miR-328、miR-335-3p、miR-374*、miR-429、miR-455*、miR-296-5p、miR-298、miR-29a*、miR-30b*、miR-30c-1*、miR-31*、miR-196b、miR-197、miR-222、miR-24-1*、miR-291b-5p改善肝脏脂代谢鞣花单宁[79]miR-302a*、miR-194、miR-433、miR-424、miR-510、miR-519e、miR-518f*-526a、miR-452、miR-518c*、miR-512-5p、miR-513、miR-370、miR-526b、miR-200a*、let-7i、let-7f、miR-299-3p、miR-542-3p、let-7d、let-7c、miR-373*、miR-525、let-7e、miR-346、miR-let-7a抑制癌细胞增殖下调Nrf2、HO、p65抗炎症葡萄白芦藜醇[90]下调TNFα抗炎症异鼠李素[91]下调TNFα抗炎症石榴多酚[91]下调Akt2抗炎症槲皮素[91]下调TNFα抗炎症石榴多酚[92]下调AKT抗炎症白藜芦醇[93]下调抗炎症1,25-二羟基维生素D[94]下调NF-κB抗炎症葡萄白藜芦醇[90]miR-34下调TNFα、IL-1β调控胰岛细胞功能玫瑰茄多酚提取物[84]miR-103上调Pank1抑制甘油三酯储存、乙酰辅酶A代谢共轭亚油酸[95]miR-107下调CEBPα、Cpt1b、Ucp2调控脂肪酸代谢玫瑰茄多酚[84]上调Pank1抑制甘油三酯储存、乙酰辅酶A代谢VD[96]miR-15a、miR-20b、miR-181c上调改善糖尿病葡萄白藜芦醇[90]miR-181上调TNFα、IL-1β抗炎症姜黄素[97]miR-199下调MMP-13、TIMP-1改善肝脏纤维化miR-200下调MMP-13、TIMP-1非瑟酮[98]miR-378下调SREBP-1、SCD1、FASN、PGC-1β、NRF-1改善脂肪酸氧化、脂肪生成儿茶素[99]let-7a上调Ras改善葡萄糖代谢、胰岛素抵抗儿茶素[99]let-7b上调K-Ras改善葡萄糖代谢、胰岛素抵抗抗炎症葡萄籽白藜芦醇[80]miR-1224-3p、miR-197和miR-532-3p上调抑制炎症、肥胖、胰岛素抵抗miR-30b*下调葡萄籽原花青素[81]下调Fas抑制脂肪酸合成甘草黄酮[82]miR-122上调PTP1B改善葡萄糖代谢甲基缺乏饮食[83]下调预防肝癌玫瑰茄多酚[84]上调Fas、SREBP1抑制脂肪酸合成槲皮素[85]上调AOAH改善类脂物代谢VE缺陷饮食[86]miR-122a、miR-125b下调抑制类脂代谢、炎症可可粉原花青素[80]miR-30b下调TGFβ、RELA、FADS2抑制炎症、调控NF-κB和PPAR信号通路葡萄籽原花青素[87]TGFβ、RELA、FADS2葡萄籽原花青素[81]miR-33下调Abca1增加胆固醇运输槲皮素[85]miR-125上调抑制炎症VE缺陷饮食[86]下调TNFα抗炎症巴西莓和葡萄多酚[87]miR-126上调VCAM-1改善氧化应激、炎症冬青叶黄酮[88]miR-146a上调IRAK1、TRAF6抗炎症异硫氰酸烯丙酯[89]miR-155葡萄籽白藜芦醇[90]miR-21上调TNFα、IL-1β番茄红素[100]FABP7改善肝脏脂代谢油酸[101]miR-21上调PTEN抑制肝脏疾病维甲酸[102]抗炎症雷公藤红素[103]miR-223上调Maspin、IL1β、ICAM-1、PLAT抑制NF-κB、改善胰岛素抵抗和炎症反应丹皮酚[104]miR-126上调抑制PI3K/Akt/NF-κB信号通路的转导、降低MC向ox-LDL损伤VEC的黏附ω-3多不饱和脂肪酸[105]miR-146b下调Smad4缓解慢性炎症天麦消渴片[106]多个miRNA18 个上调,3 个下调改善胰岛素抵抗、降血糖雷公藤多苷[107]miR-21下调改善细胞纤维化程度丹酚酸B[108]miR-106b下调通过TGF-β1/Smads信号通路阻止HK-2细胞EMT
目前,miRNA已经作为一种新兴的治疗技术进入多种疾病的临床实验阶段。2011年美国Santaris Pharma A/S公司报道了第1个进入临床实验的miRNA——miRNA-122,用于治疗慢性丙型病毒性肝炎;2013年5月美国食品药品监督管理局(food and drug administration,FDA)批准Mirna Therapeutics公司进行miRNA-34治疗肝癌的临床实验,这是进入临床实验治疗癌症的第1个miRNA;2015年11月13日,miRagen治疗公司进行MRG-106(microRNA-155的拮抗剂)和MRG-201(microRNA-29b的激动剂)的临床研究,分别用于治疗恶性造血系统疾病和病理性纤维化疾病。但是,利用人工合成的miRNA来调控体内基因表达的新兴疗法,可能存在很多潜在的安全问题:1)每个细胞miRNA都能够调控数上百种基因的表达,因此对miRNA途径,哪怕只有微小的扰动也会造成非常严重的后果;2)因现有模拟或干扰miRNA研究多基于细胞株及动物实验,所用的miRNA浓度并非正常生理条件下的人体内的miRNA浓度,所以其具体相关性功能还需进一步验证;3)miRNA功能不需要完全互补,一个miRNA可同时作用于多个靶基因,在抑制某种疾病的同时,需排除不会对人体产生其他的不良影响。而天然产物营养不仅可以有效调控机体miRNA的表达和功能,改善代谢综合征等慢性病的发生和发展,并且具有较高的安全性,也许会是将来的研究热点。
5 结 语
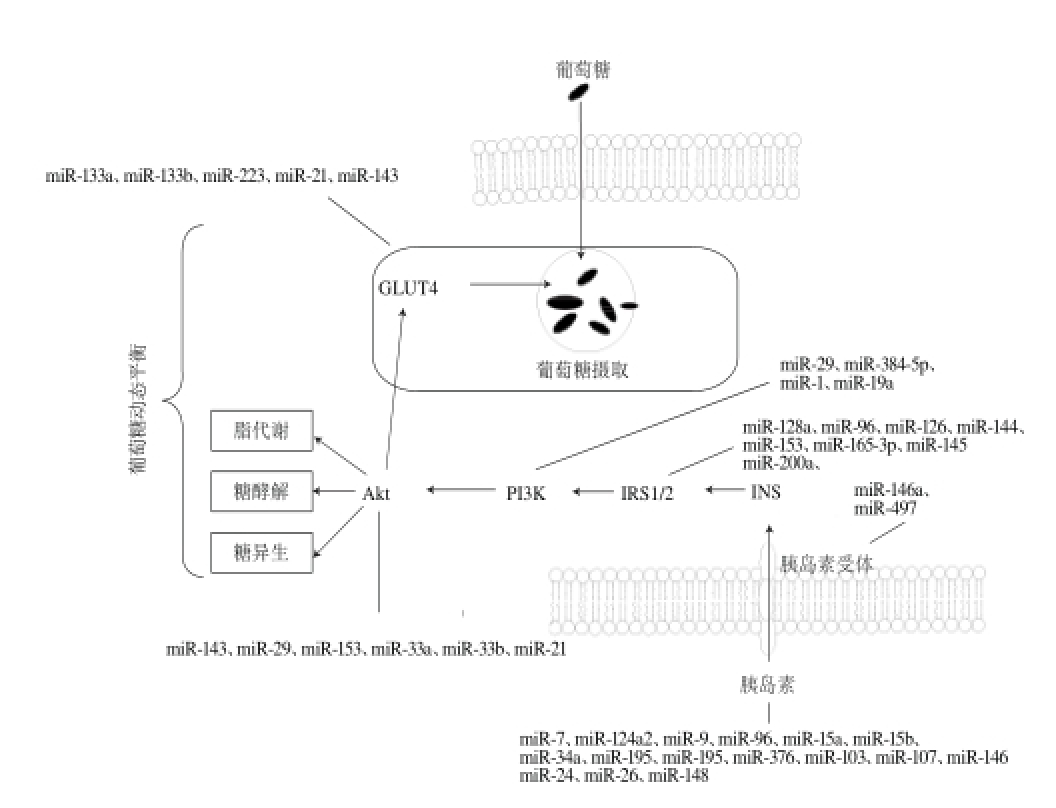
图1 miRNA在Ⅱ型糖尿病及肥胖等代谢综合征中的调控网络
Fig.1 miRNA regulatory networks in t ype Ⅱ diabetes, obesity and other metabolic syndromes
miRNA具有多种病理生理学功能,参与调控脂肪组织损伤、糖脂代谢、胰岛素抵抗,在糖尿病和肥胖等多种代谢疾病中具有至关重要的作用(图1)。虽然已有大量研究表明miRNA在代谢综合征中具有调控作用,但是却只有少数的miRNA被证明只与糖尿病相关或者只和肥胖有关,而那些在多种疾病中都发生差异性表达的miRNA常常会混淆研究者对其功能的判断,因此仍需要深入探讨研究miRNA的生物学功能,正确评价其在各种代谢综合征中的调控作用。
最近,miRNA作为健康与疾病的信号分子及生物标记物已逐渐成为研究热点,血液中的循环并稳定存在的miRNA可以作为生物标记物,胞外miRNA可以作为各种代谢疾病的分类依据,但是miRNA具有显著的个体差异,因 此其临床应用价值仍需要慎重判断。另外,大量动物及细胞研究已经表明饮食可以调控miRNA的表达和功能,为代谢综合征提供了安全有效的治疗策略,但是缺乏定性研究。因此,需要一些功能性研究揭示饮食如何参与并调控miRNA及其靶基因的表达。
参考文献:
[1] 董英, 钱希文, 白娟, 等. 苦瓜改善胰岛素抵抗功能与作用机制研究进展[J]. 食品科学, 2013, 34(21): 369-374. DOI:10.7506/spkx1002-6630-201321073.
[2] HALCOX J, MISRA A. Type 2 diabetes mellitus, metabolic syndrome, and mixed dyslipidemia: how similar, how different, and how to treat?[J]. Metabolic Syndrome & Related Disorders, 2015, 13(1): 1-21. DOI:10.1089/met.2014.0049.
[3] 黄建权, 王观春, 由凯. 肥胖和代谢综合征的药物治疗与研究进展[J].药品评价, 2013(9): 25-32.
[4] EVA V R. The art of microRNA research[J]. Circulation Research, 2011, 108(2): 219-234. DOI:10.1161/CIRCRESAHA.110.227496.
[5] 虞桂, 王阶. miRNA及其调控网络与中医治病求本机制研究[J]. 中华中医药杂志, 2012(11): 2789-2791.
[6] HARVEY A L. Plant natural products in anti-diabetic drug discovery[J]. Current Organic Chemistry, 2010, 14(16): 1670-1677. DOI:10.2174/138527210792927681.
[7] ROSS S A, DAVIS C D. The emerging role of microRNA and nutrition in modulating health and disease[J]. Annual Review of Nutrition, 2014, 34: 305-336. DOI:10.1146/annurev-nutr-071813-105729.
[8] HERRERA B M, LOCKSTONE H E, TAYLOR J M, et al. Global microRNA expression prof i les in insulin target tissues in a spontaneous rat model of type 2 diabetes[J]. Diabetologia, 2010, 53(6): 1099-1109. DOI:10.1007/s00125-010-1667-2.
[9] LI X L. MiR-375, a microRNA related to diabetes[J]. Gene, 2013, 533(1): 1-4. DOI:10.1016/j.gene.2013.09.105.
[10] ZHU L, CHEN L, SHI C M, et al. MiR-335, an adipogenesis-related microRNA, is involved in adipose tissue inflammation[J]. Cell Biochemistry & Biophysics, 2014, 68(2): 283-290. DOI:10.1007/ s12013-013-9708-3.
[11] KILIC I D, DODURGA Y, ULUDAG B, et al. MicroRNA-143 and -223 in obesity[J]. Gene, 2015, 560(2): 140-142. DOI:10.1016/j.gene.2015.01.048.
[12] GAO Song, WASSLER M, ZHANG Lulu, et al. MicroRNA-133a regulates insulin-like growth factor-1 receptor expression and vascular smooth muscle cell proliferation in murine atherosclerosis[J]. Atherosclerosis, 2014, 232(1): 171-179. DOI:10.1016/ j.atherosclerosis.2013.11.029.
[13] KULLMANN A, WEBER P S, BISHOP J B, et al. Equine insulin receptor and insulin-like growth factor-1 receptor expression in digital lamellar tissue and insulin target tissues[J]. Equine Veterinary Journal, 2015, 48(5): 626-632. DOI:10.1111/evj.12474.
[14] SUN C Z, HUANG F Z, LIU X Y, et al. MiR-21 regulates triglyceride and cholesterol metabolism in non-alcoholic fatty liver disease by targeting HMGCR[J]. International Journal of Molecular Medicine, 2015, 35(3): 847-853. DOI:10.3892/ijmm.2015.2076.
[15] ESGUERRA J L S, CAROLINE B, CILIO C M, et al. Differential glucose-regulation of microRNA in pancreatic islets of non-obese type 2 diabetes model Goto-Kakizaki rat[J]. PLoS ONE, 2011, 6(4): e18613. DOI:10.1371/journal.pone.0018613.
[16] ARNER E, MEJHERT N, KULYTÉ A, et al. Adipose tissue microRNA as regulators of CCL2 production in human obesity[J]. Diabetes, 2012, 61(8): 1986-93. DOI:10.2337/db11-1508.
[17] CAPOBIANCO V, NARDELLI C, FERRIGNO M, et al. miRNA and protein expression prof i les of visceral adipose tissue reveal miR-141/ YWHAG and miR-520e/RAB11A as two potential miRNA/protein target pairs associated with severe obesity[J]. Journal of Proteome Research, 2012, 11(6): 3358-3369. DOI:10.1021/pr300152z.
[18] DIAWARA M R, HUE C, WILDER S P, et al. Adaptive expression of microRNA-125a in adipose tissue in response to obesity in mice and men[J]. PLoS ONE, 2014, 9(3): e91375-e91375. DOI:10.1371/journal. pone.0091375.
[19] CHEN Ling, DAI Yongmei, JI Chenbo, et al. MiR-146b is a regulator of human visceral preadipocyte proliferation and differentiation and its expression is altered in human obesity[J]. Molecular and Cellular Endocrinology, 2014, 393(1/2): 65-74.
[20] 刁雪红, 申锷, 胡兵, 等. 糖尿病小鼠心肌组织microRNA表达谱分析[J]. 上海交通大学学报(医学版), 2010, 30(10): 1194-1198.
[21] TALI A S, LIA K, SHARON K R, et al. The promoter of the primiR-375 gene directs expression selectively to the endocrine pancreas[J]. PLoS ONE, 2009, 4(4): 5033. DOI:10.1371/journal. pone.0005033.
[22] El OUAAMARI A, BAROUKH N, MARTENS G A, et al. miR-375 targets 3'-phosphoinositide-dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic beta-cells[J]. Diabetes, 2008, 57(10): 2708-2717. DOI:10.2337/db07-1614.
[23] POY M N, LENA E, JAN K, et al. A pancreatic islet-specific microRNA regulates insulin secretion[J]. Nature, 2004, 432: 226-230. DOI:10.1038/nature03076.
[24] MEERSON A, TRAURIG M, OSSOWSKI V, et al. Human adipose microRNA-221 is upregulated in obesity and affects fat metabolism downstream of leptin and TNF-α[J]. Diabetologia, 2013, 56(9): 1971-1979. DOI:10.1007/s00125-013-2950-9.
[25] CHOU W W, WANG W T, LIAO Y C, et al. Decreased microRNA-221 is associated with high levels of TNF-α in human adipose tissue-derived mesenchymal stem cells from obese woman[J]. Cellular Physiology & Biochemistry, 2013, 32(1): 127-137. DOI:10.1159/000350131.
[26] MENG Shu, CAO Jiatian, ZHANG B, et al. Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene Spred-1[J]. Journal of Molecular & Cellular Cardiology, 2012, 53(1): 64-72. DOI:10.1016/ j.yjmcc.2012.04.003.
[27] ZHANG Tao, LÜ Chunfang, LI Liling, et al. Plasma miR-126 is a potential biomarker for early prediction of type 2 diabetes mellitus in susceptible individuals[J]. Biomed Research International, 2013(4): 761617. DOI:10.1155/2013/761617.
[28] YANIV L, EHUD B, REUT A F, et al. RNA-binding protein PTB and microRNA-221 coregulate AdipoR1 translation and adiponectin signaling[J]. Diabetes, 2013, 63(2): 433-445. DOI:10.2337/db13-1032.
[29] BAROUKH N, RAVIER M A, LODER M K, et al. MicroRNA-124a regulates Foxa2 expression and intracellular signaling in pancreatic beta-cell lines[J]. Journal of Biological Chemistry, 2007, 282(27): 19575-19588.
[30] LOVIS P, GATTESCO S R. Regulation of the expression of components of the exocytotic machinery of insulin-secreting cells by microRNA[J]. Biological Chemistry, 2008, 389(3): 305-312. DOI:10.1515/BC.2008.026.
[31] VALÉRIE P, AMAR A, VÉRONIQUE P M, et al. MicroRNA-9 controls the expression of Granuphilin/Slp4 and the secretory response of insulin-producing cells[J]. Journal of Biological Chemistry, 2006, 281(37): 26932-26942. DOI:10.1074/jbc.M601225200.
[32] HE Aibin, ZHU Liuluan, GUPTA N, et al. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes[J]. Molecular Endocrinology, 2007, 21(11): 2785-2794. DOI:10.1210/me.2007-0167.
[33] MIRKO T, JEAN H, JÜRGEN S, et al. MicroRNA 103 and 107 regulate insulin sensitivity[J]. Nature, 2011, 474: 649-653. DOI:10.1038/nature10112.
[34] CHEN Lin, HOU Jia, YE Lanfeng, et al. MicroRNA-143 regulates adipogenesis by modulating the MAP2K5-ERK5 signaling[J]. Scienti fi c Reports, 2014, 4(1): 3819-3819. DOI:10.1038/srep03819.
[35] LORENTE-CEBRIÁN S, MEJHERT N, KULYTÉ A, et al. MicroRNA regulate human adipocyte lipolysis: effects of miR-145 are linked to TNF-α[J]. PLoS ONE, 2014, 9(1): e86800. DOI:10.1371/ journal.pone.0086800.
[36] 史春梅, 徐广峰, 季晨博, 等. miR-26b过表达对不同时间点人脂肪细胞分泌脂因子的影响[J]. 临床儿科杂志, 2013(10): 914-916. DOI:10.3969/j.issn.1000-3606.2013.10.004.
[37] BALASUBRAMANYAM M, ARAVIND S, GOKULAKRISHNAN K, et al. Impaired miR-146a expression links subclinical inflammation and insulin resistance in Type 2 diabetes[J]. Molecular and Cellular Biochemistry, 2011, 351(1/2): 197-205. DOI:10.1007/s11010-011-0727-3.
[38] SALAS-PÉREZ F, CODNER E, VALENCIA E, et al. MicroRNA miR-21a and miR-93 are down regulated in peripheral blood mononuclear cells (PBMCs) from patients with type 1 diabetes[J]. Immunobiology, 2013, 218(5): 733-737. DOI:10.1016/ j.imbio.2012.08.276.
[39] ZHONG X, CHUNG A C K, CHEN H Y, et al. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes[J]. Diabetologia, 2013, 56(3): 663-674. DOI:10.1007/s00125-012-2804-x.
[40] FENG Biao, CAO Yanan, CHEN Shali, et al. MiRNA-1 regulates endothelin-1 in diabetes[J]. Life Sciences, 2014, 118(2): 18-23. DOI:10.1016/j.lfs.2013.12.199.
[41] KATO M, ZHANG J, WANG M, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors[J]. Proceedings of the National Academy of Sciences, 2007, 104(9): 3432-3437. DOI:10.1073/pnas.0611192104.
[42] CHEN Shali, PUTHANVEETIL P, FENG Biao, et al. Cardiac miR-133a overexpression prevents early cardiac fi brosis in diabetes[J]. Journal of Cellular & Molecular Medicine. 2014, 18(3): 415-421. DOI:10.1111/ jcmm.12218.
[43] COLLARES C V, EVANGELISTA A F, XAVIER D J, et al. Identifying common and specif i c microRNA expressed in peripheral blood mononuclear cell of type 1, type 2, and gestational diabetes mellitus patients[J]. BMC Research Notes, 2013, 6(1): 1-15. DOI:10.1186/1756-0500-6-491.
[44] KONG Lei, ZHU Junjie, HAN Wenxia, et al. Significance of serum microRNA in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study[J]. Acta Diabetologica, 2011, 48(1): 61-69. DOI:10.1007/s00592-010-0226-0.
[45] WANG X, SUNDQUIST J, ZÖLLER B, et al. Determination of 14 circulating microRNA in Swedes and Iraqis with and without diabetes mellitus type 2[J]. PLoS ONE, 2014, 9(1): e86792. DOI:10.1371/ journal.pone.0086792.
[46] 叶青. 脂代谢相关miRNA靶基因结合区域多态性与代谢综合征及其组分的分子流行病学研究[D]. 南京: 南京医科大学, 2013: 39-56.
[47] MARTINELLI R, NARDELLI C, PILONE V, et al. miR-519d Overexpression is associated with human obesity[J]. Obesity, 2010, 18(11): 2170-2176. DOI:10.1038/oby.2009.474.
[48] HENEGHAN H M, MILLER N , MCANENA O J, et al. Differential miRNA expression in omental adipose tissue and in the circulation of obese patients identifies novel metabolic biomarkers[J]. Journal of Clinical Endocrinology & Metabolism, 2011, 96(5): 846-850. DOI:10.1210/jc.2010-2701.
[49] KELLER P, GBURCIK V, PETROVIC N, et al. Gene-chip studies of adipogenesis-regulated microRNA in mouse primary adipocytes and human obesity[J]. BMC Endocrine Disorders, 2011, 11(1): 1-11. DOI:10.1186/1472-6823-11-7.
[50] ORTEGA F J, MORENO-NAVARRETE J M, GERARD P, et al. MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation[J]. PLoS ONE, 2010, 5(2): e9022. DOI:10.1371/journal.pone.0009022.
[51] ZHUANG Guoqing, MENG Cong, GUO Xin, et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation[J]. Circulation, 2012, 125(23): 2892-2903. DOI:10.1161/CIRCULATIONAHA.111.087817.
[52] FU Ting, CHOI S E, KIM D H, et al. Aberrantly elevated microRNA-34a in obesity attenuates hepatic responses to FGF19 by targeting a membrane coreceptor β-Klotho[J]. Proceedings of the National Academy of Sciences, 2012, 109(40): 16137-16142. DOI:10.1073/ pnas.1205951109.
[53] PAN Shifeng, ZHENG Yating, ZHAO Ruqian, et al. MicroRNA-130b and microRNA-374b mediate the effect of maternal dietary protein on offspring lipid metabolism in Meishan pigs[J]. British Journal of Nutrition, 2013, 109(10): 1-8. DOI:10.1017/S0007114512003728.
[54] WANG Yucheng, LI Yuying, WANG Xinyi, et al. Circulating miR-130b mediates metabolic crosstalk between fat and muscle in overweight/obesity[J]. Diabetologia, 2013, 56(10): 2275-2285. DOI:10.1007/s00125-013-2996-8.
[55] HULSMANS M, van DOOREN E, MATHIEU C, et al. Decrease of miR-146b-5p in monocytes during obesity is associated with loss of the anti-inf l ammatory but not insulin signaling action of adiponectin[J]. PLoS ONE, 2012, 7(2): e32794. DOI:10.1371/journal.pone.0032794.
[56] CHOI S E, FU T, SEOK S, et al. Elevated microRNA-34a in obesity reduces NAD+levels and SIRT1 activity by directly targeting NAMPT[J]. Aging Cell, 2013, 12(6): 1062-1072. DOI:10.1111/ acel.12135.
[57] BENATTI R O, MELO A M, BORGES F O, et al. Maternal highfat diet consumption modulates hepatic lipid metabolism and microRNA-122 (miR-122) and microRNA-370 (miR-370) expression in offspring[J]. British Journal of Nutrition, 2014, 111(12): 2112-2122. DOI:10.1017/S0007114514000579.
[58] FOLEY N H, O'NEILL L A. MiR-107: a toll-like receptor-regulated miRNA dysregulated in obesity and type Ⅱ diabetes[J]. Journal of Leukocyte Biology, 2012, 92(3): 521-527. DOI:10.1189/jlb.0312160.
[59] NAKANISHI N, NAKAGAWA Y, TOKUSHIGE N. The up-regulation of microRNA-335 is associated with lipid metabolism in liver and white adipose tissue of genetically obese mice[J]. Biochemical & Biophysical Research Communications, 2009, 385(4): 492-496. DOI:10.1016/j.bbrc.2009.05.058.
[60] KORNFELD J W, BAITZEL C, KÖNNER A C, et al. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b[J]. Nature, 2013, 494: 111-115. DOI:10.1038/ nature11793.
[61] JORDAN S D, MARKUS K, WILLMES D M, et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism[J]. Nature Cell Biology, 2011, 13(4): 434-446. DOI:10.1038/ncb2211.
[62] KIM C, LEE H, CHO Y M, et al. TNFα-induced miR-130 resulted in adipocyte dysfunction during obesity-related inflammation[J]. Febs Letters, 2013, 587(23): 3853-3858. DOI:10.1016/ j.febslet.2013.10.018.
[63] ORTEGA F J, MERCADER J M, JOSÉ MARÍA M N, et al. Prof i ling of circulating microRNA reveals common microRNA linked to type 2 diabetes that change with insulin sensitization[J]. Diabetes Care, 2014, 37(5): 1375-1383. DOI:10.2337/dc13-1847.
[64] ORTEGA F J, MERCADER J M, CATALÁN V, et al. Targeting the circulating microRNA signature of obesity[J]. Clinical Chemistry, 2013, 59(5): 781-792. DOI:10.1373/clinchem.2012.195776.
[65] ANNA P P, ORTEGA F J, MERCADER J M, et al. Changes in circulating microRNA are associated with childhood obesity[J]. Journal of Clinical Endocrinology and Metabolism, 2013, 98(10): 1655-1660. DOI:10.1210/jc.2013-1496.
[66] CARRER M, LIU N, GRUETER C E, et al. Control of mitochondrial metabolism and systemic energy homeostasis by microRNA 378 and 378*[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(38): 15330-15335. DOI:10.1073/ pnas.1207605109.
[67] 张政, 罗勇军, 彭惠民, 等. 早期糖尿病肾病相关微小RNA在小鼠肾脏的表达变化[J]. 第三军医大学学报, 2010, 32(13): 1383-1386.
[68] ZHANG Z, PENG H M, CHEN J X, et al. MicroRNA-21 protects from mesangial cell proliferation induced by diabetic nephropathy in db/db mice[J]. Febs Letters, 2009, 583(12): 2009-2014. DOI:10.1016/ j.febslet.2009.05.021.
[69] CHENG Y H, LIU X J, YANG J, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation[J]. Circulation Research, 2009, 105(2): 158-166. DOI:10.1161/CIRCRESAHA.109.197517.
[70] ANDREA C, MARCO M, CHRISTINE V, et al. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia[J]. Circulation, 2011, 123(3): 282-291. DOI:10.1161/ CIRCULATIONAHA.110.952325.
[71] FERREIRA L R P, AMANDA F F, SANTOS R H B, et al. MicroRNA miR-1, miR-133a, miR-133b, miR-208a and miR-208b are dysregulated in Chronic Chagas disease Cardiomyopathy[J]. International Journal of Cardiology, 2014, 175(3): 409-417. DOI:10.1016/j.ijcard.2014.05.019.
[72] TURCHINOVICH A, WEIZ L, BURWINKEL B. Extracellular miRNAs: the mystery of their origin and function[J]. Trends in Biochemical Sciences, 2012, 37(11): 460-465. DOI:10.1016/ j.tibs.2012.08.003.
[73] ZHANG Lin, HOU Dongxia, CHEN Xi, et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA[J]. Cell Research, 2012, 22(1): 107-126. DOI:10.1038/cr.2011.158.
[74] WANG K, LI H, YUAN Y, et al. The complex exogenous RNA spectra in human plasma: an interface with human gut biota?[J]. PLoS ONE, 2012, 7(12): 1354-1357. DOI:10.1371/journal.pone.0051009.
[75] KOSAKA N, IZUMI H, SEKINE K, et al. MicroRNA as a new immune-regulatory agent in breast milk[J]. Silence, 2010, 1(1): 7. DOI:10.1186/1758-907X-1-7.
[76] TITLE A C, DENZLER R, STOFFEL M. Uptake and function studies of maternal milk-derived microRNAs*[J]. Journal of Biological Chemistry, 2015, 290(39): 23680-23691. DOI:10.1074/jbc. M115.676734.
[77] DRAGAN M, CHRISTIANE D, ERWAN G, et al. Modulation of miRNA expression by dietary polyphenols in apoE def i cient mice: a new mechanism of the action of polyphenols[J]. PLoS ONE, 2012, 7(1): 156-167. DOI:10.1371/journal.pone.0029837.
[78] NOLTE H E N M, van ROOIJ E, BUSHELL M, et al. The role of microRNA in nutritional control[J]. Journal of Internal Medicine, 2015, 278(2): 99-109. DOI:10.1111/joim.12372.
[79] WEN X Y, WU S Y, LI Z Q, et al. Ellagitannin (BJA3121), an antiproliferative natural polyphenol compound, can regulate the expression of MiRNAs in HepG 2 cancer cells[J]. Phytotherapy Research, 2009, 23(6): 778-784. DOI:10.1002/ptr.2616.
[80] AROLA-ARNAL A, BLADÉC. Proanthocyanidins modulate microRNA expression in human HepG2 cells[J]. PLoS ONE, 2011, 6(10): 148. DOI:10.1371/ journal.pone.0025982.
[81] BASELGA-ESCUDERO L, BLADÉ C, RIBAS-LATRE A, et al. Grape seed proanthocyanidins repress the hepatic lipid regulators miR-33 and miR-122 in rats[J]. Molecular Nutrition & Food Research, 2012, 56(11): 1636-1646. DOI:10.1002/mnfr.201200237.
[82] YANG Y M, SEO S Y, KIM T H, et al. Decrease of microRNA-122 causes hepatic insulin resistance by inducing protein tyrosine phosphatase 1B, which is reversed by licorice flavonoid[J]. Hepatology, 2012, 56(6): 2209-2220. DOI:10.1002/hep.25912.
[83] KUTAY H, BAI S, DATTA J, et al. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas[J]. Journal of Cellular Biochemistry, 2006, 99(3): 671-678. DOI:10.1002/jcb.20982.
[84] JOVEN J, ESPINEL E, RULL A, et al. Plant-derived polyphenols regulate expression of miRNA paralogs miR-103/107 and miR-122 and prevent diet-induced fatty liver disease in hyperlipidemic mice[J]. Biochimica et Biophysica Acta, 2012, 1820(7): 894-899. DOI:10.1016/ j.bbagen.2012.03.020.
[85] BOESCH-SAADATMANDI C, WAGNER A E, WOLFFRAM S, et al. Effect of quercetin on inflammatory gene expression in mice liver in vivo-role of redox factor 1, miRNA-122 and miRNA-125b[J]. Pharmacological Research the Off i cial Journal of the Italian Pharmacological Society, 2012, 65(5): 523-530. DOI:10.1016/ j.phrs.2012.02.007.
[86] GAEDICKE S, ZHANG X, SCHMELZER C, et al. Vitamin E dependent microRNA regulation in rat liver[J]. Febs Letters, 2008, 582(23/24): 3542-3546. DOI:10.1016/j.febslet.2008.09.032.
[87] NORATTO G D, GABRIELA A M, TALCOTT S T, et al. Polyphenolics from aç aí (Euterpe oleracea Mart.) and red muscadine grape (Vitis rotundifolia ) protect human umbilical vascular endothelial cells (HUVEC) from glucose- and lipopolysaccharide (LPS)-induced inf l ammation and target microRNA-126[J]. Journal of Agricultural and Food Chemistry, 2011, 59(14): 7999-8012. DOI:10.1021/jf201056x.
[88] NORATTO G D, YOUNGMOK K, TALCOTT S T, et al. Flavonol-rich fractions of yaupon holly leaves (Ilex vomitoria, Aquifoliaceae) induce microRNA-146a and have anti-inflammatory and chemopreventive effects in intestinal myofibroblast CCD-18Co cells[J]. Fitoterapia, 2011, 82(4): 557-569. DOI:10.1016/j.f i tote.2011.01.013.
[89] WAGNER A E, BOESCH-SAADATMANDI C, DOSE J, et al. Antiinf l ammatory potential of allyl-isothiocyanate-role of Nrf2, NF-κB and microRNA-155[J]. Journal of Cellular and Molecular Medicine, 2012, 16(4): 836-843. DOI:10.1111/j.1582-4934.2011.01367.x.
[90] TOMÉ-CARNEIRO J, LARROSA M, YÁÑEZ-GASCÓN M J, et al. One-year supplementation with a grape extract containing resveratrol modulates inf l ammatory-related microRNA and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease[J]. Pharmacological Research, 2013, 72(3): 69-82. DOI:10.1016/j.phrs.2013.03.011.
[91] BOESCH-SAADATMANDI C, LOBODA A, WAGNER A E, et al. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: role of miR-155[J]. Journal of Nutritional Biochemistry, 2011, 22(3): 293-299. DOI:10.1016/j.jnutbio.2010.02.008.
[92] NIVEDITA B, STEPHEN T, STEPHEN S, et al. Cytotoxicity of pomegranate polyphenolics in breast cancer cells in vitro and vivo: potential role of miRNA-27a and miRNA-155 in cell survival and inf l ammation[J]. Breast Cancer Research & Treatment, 2012, 136(1): 21-34. DOI:10.1007/s10549-012-2224-0.
[93] TILI E, MICHAILLE J B, ALDER H, et al. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD[J]. Carcinogenesis, 2010, 31(9): 1561-1566. DOI:10.1093/carcin/bgq143.
[94] CHEN Y Z, LIU W C, SUN T, et al. 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages[J]. Journal of Immunology, 2013, 190(7): 3687-3695. DOI:10.4049/jimmunol.1203273.
[95] PILAR P, FRANCISCA S, ANDREU P. Expression of adipose microRNA is sensitive to dietary conjugated linoleic acid treatment in mice[J]. PLoS ONE, 2010, 5(9): e13005. DOI:10.1371/journal. pone.0013005.
[96] ZITMAN-GAL T, GREEN J, PASMANIK-CHOR M, et al. Vitamin D manipulates miR-181c, miR-20b and miR-15a in human umbilical vein endothelial cells exposed to a diabetic-like environment[J]. Cardiovascular Diabetology, 2014, 13(1): 8. DOI:10.1186/1475-2840-13-8.
[97] HASSAN Z K, Al-OLAYAN E M. Curcumin reorganizes miRNA expression in a mouse model of liver fi brosis[J]. Asian Pacif i c Journal of Cancer Prevention Apjcp, 2012, 13(13): 5405-5408. DOI:10.7314/ APJCP.2012.13.11.5405.
[98] LAM T K, STEPHANIE S, YINGDONG Z, et al. Influence of quercetin-rich food intake on microRNA expression in lung cancer tissues[J]. Cancer Epidemiology Biomarkers & Prevention, 2012, 21(12): 2176-2184. DOI:10.1158/1055-9965.EPI-12-0745.
[99] TSANG W P, KWOK T T. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells[J]. Journal of Nutritional Biochemistry, 2010, 21(2): 140-146. DOI:10.1016/ j.jnutbio.2008.12.003.
[100] AHN J, LEE H, CHANG H J, et al. Lycopene inhibits hepatic steatosis via microRNA-21-induced downregulation of fatty acid-binding protein 7 in mice fed a high-fat diet[J]. Molecular Nutrition & Food Research, 2012, 56(11): 1665-1674. DOI:10.1002/mnfr.201200182.
[101] VINCIGUERRA M, SGROI A, VEVRAT-DUREBEX C, et al. Unsaturated fatty acids inhibit the expression of tumor suppressor phosphatase and tensin homolog (PTEN) via microRNA-21 upregulation in hepatocytes[J]. Hepatology, 2009, 49(4): 1176-1184. DOI:10.1002/hep.22737.
[102] TEROAO M, FRATELLIM, KUROSAKI M, et al. Induction of miR-21 by retinoic acid in estrogen receptor-positive breast carcinoma cells[J]. Journal of Biological Chemistry, 2011, 286(5): 4027-4042. DOI:10.1074/jbc.M110.184994.
[103] 张雪. 雷公藤红素通过抑制NF-κB上调miR-223,纠正软脂酸导致的HepG2细胞糖吸收下降[D]. 银川: 宁夏医科大学, 2014: 12-41.
[104] 袁绪胜. MicroRNA-126对ox-LDL诱导血管内皮细胞黏附功能的调控及丹皮酚干预机制[D]. 合肥: 安徽中医药大学, 2013: 54-79.
[105] 郑征. Omega-3多不饱和脂肪酸通过影响miR: 146b的表达实现对炎症的调节[D]. 青岛: 青岛大学, 2013: 24-42.
[106] 张茜. 天麦消渴片通过microRNA和mRNA调控网络改善糖代谢机制研究[D]. 北京: 北京协和医学院, 2014: 74-135.
[107] 贾蓉. miRNA-21在UUO幼年大鼠肾组织中的表达趋势及药物干预的效果[D]. 太原: 山西医科大学, 2014: 12-32.
[108] 谢凤燕. 丹酚酸B阻止TGF-β1诱导的HK-2细胞转分化以及相关microRNA的变化[D]. 南京: 南京医科大学, 2012: 16-46.
Progress in Research on Natural Products in MicroRNA Regulation for Metabolic Syndrome Improvement
GUO Qin1, BAI Jie1, HE Yuxuan1, TANG Chuanhui1, CAO Qingguo1,2, DONG Ying1
(1. School of Food and Biological Engineering, Jiangsu University, Zhenjiang 212013, China; 2. Institute of Biological Engineering, Jiangsu Polytechnic College of Agriculture and Forestry, Jurong 212000, China)
Abstract:The metabolic syndrome has become a global health problem. microRNA (miRNA), a short single-stranded endogenously expressed RNA (approximately 22 nt), regulates gene expression at the post-transcriptional level. A lot of researches have shown that miRNA plays an important role in metabolic syndromes. Additionally, natural products which can be used as both food and medicine can modulate the activities and functions of miRNA to improve glucose and lipid metabolism disorders. Recent evidence described in this review highlights the signif i cant role of miRNA in metabolic syndromes and how dietary factors may inf l uence metabolic syndromes by modulating the activities and functions of miRNA.
Key words:metabolic syndrome; microRNA (miRNA); natural products
DOI:10.7506/spkx1002-6630-201709039
中图分类号:TS201.4
文献标志码:A
文章编号:1002-6630(2017)09-0239-09
引文格式:
郭钦, 白洁, 何宇轩, 等. 天然产物与microRNA调控代谢综合征的研究进展[J]. 食品科学, 2017, 38(9): 239-247. DOI:10.7506/spkx1002-6630-201709039. http://www.spkx.net.cn
GUO Qin, BAI Jie, HE Yuxuan, et al. Progress in research on natural products in microRNA regulation for metabolic syndrome improvement[J]. Food Science, 2017, 38(9): 239-247. (in Chinese with English abstract)
DOI:10.7506/spkx1002-6630-201709039. http://www.spkx.net.cn
收稿日期:2016-04-21
基金项目:国家自然科学基金面上项目(31371760);江苏省高等学校大学生创新项目(201510299072X)
作者简介:郭钦(1980—),女,副教授,博士,研究方向为食品营养与安全。E-mail:guoqin_shiyin@163.com





