Rational Design of Corynebacterium glutamicum YILW for Isoleucine Production Based on Gene Transcription and Metabolite Analysis
WEN Bing, MA Jie, LI Zhixiang, ZHANG Chenglin, XU Qingyang, CHEN Ning
*
(National and Local United Engineering Lab of Metabolic Control Fermentation Technology, College of Biotechnology, Tianjin University of Science and Technology, Tianjin 300457, China)
Abstract:This study aimed to rationally identify new targets for improving isoleucine production. The transcription levels of the key genes and intermediate metabolite levels involved in the isoleucine synthesis pathway of Corynebacterium glutamicum ATCC 13032 and C. glutamicum YILW, a isoleucine-producing strain derived from the parental strain ATCC 13032, were compared. The gene pyc was down-regulated, which might consequently lead to reduced supply of oxaloacetate. Then pyc was overexpressed in C. glutamicum YILW (denoted as YILW-1), resulting in increased oxaloacetate concentration and isoleucine production (from 1.32 to 3.32 μmol/g (m
d) and from 5.18 to 5.81g/L) but higher accumulation of lysine and intracellular 2-ketobutyrate as byproduct. The ilvBNC operon was further overexpressed in YILW-1 (denoted as YILW-2), resulting in production of up to 6.63 g/L isoleucine. To enhance exportation and consequently further increase the production of isoleucine, the isoleucine exporter genes brnE and brnF was overexpressed in YILW-2 (denoted as YILW-3), leading to increased production of isoleucine (7.31 g/L) by 10.3% as compared to that of YILW-2. The strategy resulted in 41.1% higher isoleucine production (from 5.18 to 7.31 g/L) and 40.0% higher yield (from 0.10 to 0.14 g/g glucose) together with lower by-product lelvels by YILW-3 as compared to C. glutamicum YILW. It could be concluded that overexpression of the pyc, ilvBNC operon as well as brnE and brnF based on transcription and metabolite pool analysis could signif i cantly elevate isoleucine production and decrease by-product concentration levels.
Key words:Corynebacterium glutamicum; isoleucine; metabolite pool; oxaloacetate
As one of essential amino acids, isoleucine can improve endurance and assists in the repair and rebuilding of muscle. Isoleucine has been widely used in various fi elds, including food additives, components of cosmetics and pharmaceuticals, and additives in infusion solutions and precursors of herbicides
[1-4].

The numbers are the ratios of the comparative transcription levels in C. glutamicum strain YILW vs. ATCC 13032. pyc 0.21 indicates downregulation and others indicates up-regulation. The solid lines represent metabolic conversions, the dotted line depicts feedback control, and the thick arrows indicate the increased flux by directly overexpressing the corresponding genes. The × indicates the removal of the feedback control.
Fig.1 Isoleucine biosynthesis pathway of C. glutamicum
At present, industrial production of isoleucine is mainly achieved through fermentation by Corynebacterium glutamicum
[5]. C. glutamicum synthesizes L-isoleucine in a split pathway from oxaloacetate via aspartate, threonine, ketobutyrate and 2-aceto-2-hydroxybutyrate as the main intermediates
[6-7]. In the pathway, phosphoenolpyruvate carboxylase (PEPC, encoded by ppc), pyruvate carboxylase (PC, encoded by pyc), aspartate kinase (AK, encoded by Cgl0251), homoserine dehydrogenase (HD, encoded by Cgl1183), homoserine kinase (HK, encoded by Cgl1184), threonine synthetase (TS, encoded by Cgl2220), threonine dehydrogenase (TD, encoded by ilvA) and acetohydroxy acid synthase (AHAS, encoded by ilvB and ilvN ) are the key enzymes(Fig. 1)
[8-9].
The isoleucine-producing strains currently used in industry were mainly obtained through random mutagenesis
[10]. However, such conventional methods relay on the chance to encounter desired mutants among resulting colonies that inevitably accumulate numerous unidentif i able and unwanted mutations. Moreover, unwanted changes in physiology and growth retardation may occur alongside the improvements
[11]. Recently, rational metabolic engineering has been receiving much research attention in both academia and industries as a method to overcome the disadvantages of the classical method of strain development
[12].
In this study, we took a strategy to increase isoleucine production based on transcriptional analysis of key genes involved in isoleucine synthesis and integrated with metabolite pool analysis. With the direction of the strategy, pyc and desensitized ilvBNC operon as well as isoleucine exporter brnE and brnF were rationally overexpressed, resulting in 41.1% and 40.0% increases in isoleucine production and yield by YILW-3. The design principles described in this study would be useful to construct strains for producing other similar biological products.
1 Materials and Methods
1.1 Strains, plasmids and primers
Strains, plasmids and primers used in this work were listed in Table1 and Table 2.
Table1 Strains and plasmids used in this study
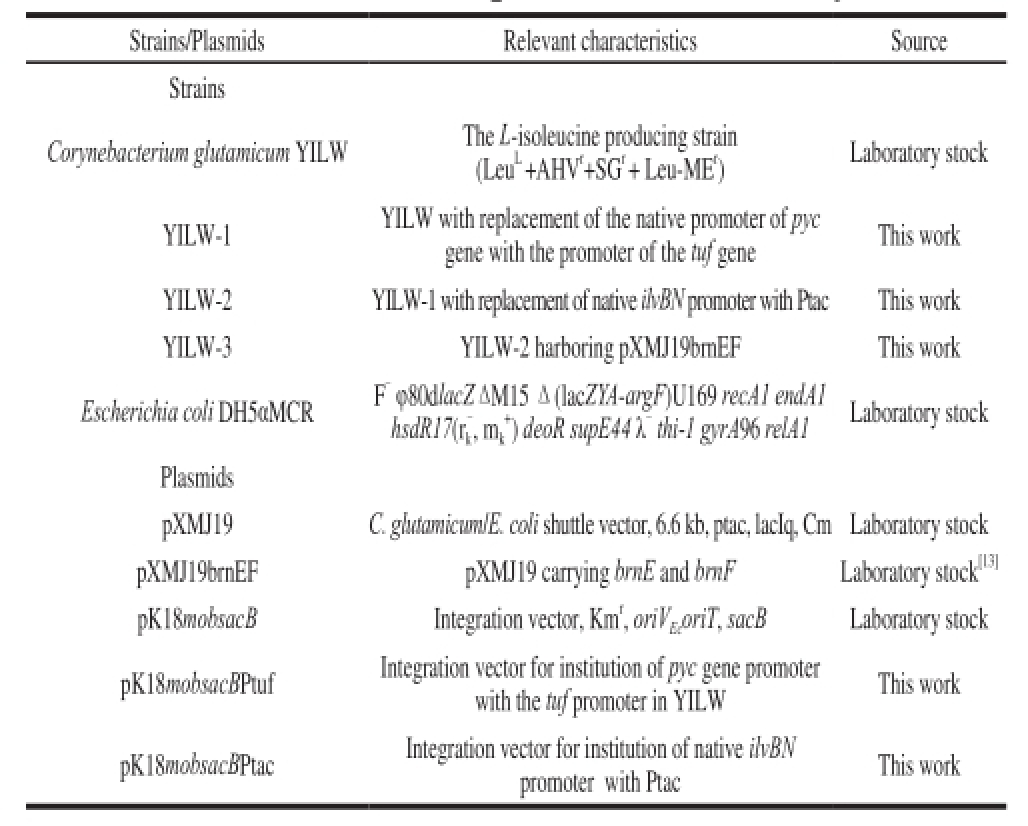
Strains/PlasmidsRelevant characteristicsSource Strains Corynebacterium glutamicum YILWThe L-isoleucine producing strain (Leu
L+AHV
r+SG
r+ Leu-ME
r)Laboratory stock YILW-1YILW with replacement of the native promoter of pyc gene with the promoter of the tuf geneThis work YILW-2YILW-1 with replacement of native ilvBN promoter with Ptac This work YILW-3YILW-2 harboring pXMJ19brnEFThis work Escherichia coli DH5αMCRF
-φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(r
k
+) deoR supE44 λ
-thi-1 gyrA96 relA1Laboratory stock Plasmids pXMJ19C. glutamicum/E. coli shuttle vector, 6.6 kb, ptac, lacIq, Cm Laboratory stock pXMJ19brnEFpXMJ19 carrying brnE and brnFLaboratory stock
[13]pK18mobsacBIntegration vector, Km
r, oriV
EcoriT, sacBLaboratory stock pK18mobsacBPtuf Integration vector for institution of pyc gene promoter with the tuf promoter in YILWThis work pK18mobsacBPtacIntegration vector for institution of native ilvBN promoter with PtacThis work
-, m
k
Table2 Primers used in this study

Note: Recognition sites are underlined and restriction enzymes are shown in parentheses.
?
1.2 Instruments and equipments
One Step SYBR
®PrimeScript™ RT-PCR Kit was purchased from Takara (Japan); SBA biosensor analyzer was obtained from Institute of Biology of Shandong Provincial Academy of Sciences (Shandong, China); high performance liquid chromatography (HPLC) was purchased from Agilent (Santa Clara, CA USA).
1.3 Methods
1.3.1 Media and growth conditions
Lysogeny Broth (LB) medium supplemented with corresponding antibiotics was used for growth of E. coli and C. glutamicum strains.
C. glutamicum cells was inoculated from seed culture (glucose 30 g/L, yeast extract 5 g/L, (NH
4)
2SO
43 g/L, KH
2PO
4·3H
2O 1.5 g/L, MgSO
4·7H
2O 0.6 g/L, FeSO
4·7H
2O 0.01 g/L, MnSO
4·H
2O 0.01 g/L, corn steep liquor 30 mL/L, soybean hydrolysate 30 mL/L) and cultured to exponential growth period (for quantitative real time-polymerase chain reaction (RT-qPCR) and intracellular metabolites detection) or to 48 h (for isoleucine fermentation) in 27 mL of fermentation medium (glucose 80 g/L, (NH
4)
2SO
44 g/L, FeSO
4·7H
2O 0.015 g/L, MgSO
4·7H
2O 0.5 g/L, MnSO
4·H
2O 0.015 g/L, KH
2PO
4·3H
2O 1.5 g/L, K
2HPO
4·3H
2O 3 g/L, biotin 100 μg/L, VB
15 mg/L, soybean hydrolysate 20 mL/L, and corn syrup 15 mL/L) in 500-mL shake fl asks with 10 % (V/V) inoculum size at 35 ℃ with 200 r/min
[12].
1.3.2 Quantitative RT-PCR
Total RNA was isolated from C. glutamicum cells and transcription levels of selected genes were measured by quantitative RT-PCR according to the manufacturer instructions of One Step SYBR
®PrimeScript™ RT-PCR Kit. Data were analyzed using the 2
-Δ
ΔCTmethod
[14]and 16S rDNA was used as the internal control.
1.3.3 Measurement of metabolite concentrations
5 mL of samples were injected into 15 mL of quenching solutions (70 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) in 60% aqueous methanol (V/V), -50 ℃) and centrifuged at 6 000×g at -20 ℃. Cell pellets were collected and resuspended in 35% (V/V) perchloric acid. After one freeze (-80 ℃)-thaw cycle, the sample was neutralized with 5 mmol/L K
2CO
3and the precipitate was removed by another centrifugation. The resulting supernatants were stored at -80 ℃ until analysis
[15]. Intracellular oxaloacetate, aspartate, threonine, 2-ketobutyrate and isoleucine were detected by HPLC.
1.3.4 Constructions of YILW-1, YILW-2 and YILW-3 strains
Fragment, in which the promoter of tuf gene
[16]wasflanked by regions upstream and downstream the native promoter of pyc was generated in two rounds of overlap PCR using primers indicated in Table2 (Fig. 2). Fragment containing regions upstream and downstream of the native promoter of ilvBNC operon as well as tac promoter
[17]was obtained by the same way. The fragments were then inserted into pK18mobsacB after being digested with Xba Ⅰ/Hind Ⅲand Hind Ⅲ/BamH Ⅰ, respectively, using E. coli DH5αMCR as host. The plasmids were designated as pK18mobsacBPtuf and pK18mobsacBPtac, respectively (Fig. 2).

Fig.2 Construction of YILW-2
The plasmid pK18mobsacBPtuf was electroporated into C. glutamicum YILW where Ptuf replaced the native promoter of pyc was constructed by two-step double homologous as reported (Fig. 2)
[15,18]. Strain YILW-2 where tac promoter replaced the native promoter in YILW-1 was constructed with the same method using pK18mobsacBPtac. Finally, pXMJ19brnEF was introduced into YILW-2, resulting YILW-3.
1.3.5 Analytical procedure
Cell growth was monitored by measuring OD
600
nmand was converted to the corresponding cell dry weight (m
d/ (g/L)=0.24×OD
600
nm-0.01). Glucose concentration was determined by biosensor analyzer. Amino acids were analyzed by precolumn derivatization with 2,4-fluoro-dinitrobenzene and detected by HPLC with an Agilent C
18column (150 mm×4.6 mm, 3.5 μm). Elution was performed using a gradient of 50% acetonitrile (V/V)/50mmol/L (CH
3COONa), and injected at a constant flow rate of 1.0 mL/min. UV absorption at 360 nm was measured and the column temperature was maintained at 33 ℃.
1.4 Statistical analysis
All the experiments were repeated three times, and data were presented as
 ±s (n=3). One-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test was used to determine significant difference, and the statistical signi fi cance was de fi ned as P≤0.05.
±s (n=3). One-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test was used to determine significant difference, and the statistical signi fi cance was de fi ned as P≤0.05.
2 Results and Analysis
2.1 Analysis of transcriptional levels of key genes and intermediate metabolites involved in isoleucine synthesis in C. glutamicum strains
Transcriptional levels of key genes (ppc, pyc, Cgl0251, Cgl1183, Cgl1184, Cgl2220, ilvA, and ilvB) involved in isoleucine synthesis in C. glutamicum YILW and C. glutamicum ATCC 13032 were detected by quantitative RT-PCR. Results showed that transcription levels of all the genes examined except pyc were signif i cantly up-regulated in C. glutamicum YILW as compared to those in C. glutamicum ATCC 13032 (Fig. 1).
In C. glutamicum, anaplerotic pathways catalyzed by PEPC and PC is the main source of oxaloacetate, which is the starting point of isoleucine synthesis in C. glutamicum. Although transcription level of ppc was up-regulated, it appears that PC is predominant during synthesis process of L-aspartate family amino acids
[19-21]. So it can be assumed that the low expression of pyc may lead to a short supply of oxaloacetate in C. glutamicum YILW.
Table3 Intracellular metabolite pool of C. glutamicum strains

Note: Δ. Significantly different as compared with ATCC13032 (P<0.05); *. Significantly different as compared with respective starting strains (P<0.05), e.g. intracellular oxaloacetate concentration of YILW-1 was significantly higher than that of its starting strain YILW and intracellular isoleucine concentration of YILW-2 was signif i cantly higher than that of its starting strain YILW-1; —. Never detected.
?
Subsequently, the concentrations of intracellular oxaloacetate together with aspartate, threonine and 2-ketobutyrate as main intermediate metabolites weredetected. It was found that oxaloacetate concentration in C. glutamicum YILW (1.32 μmol/g) was 37.4% lower than that in C. glutamicum ATCC 13032 (2.11 μmol/g, Table 3). However, concentrations of the other metabolites were similar in the two strains. So it could be deduced that a smaller oxaloacetate pool caused by down-regulated pyc is the metabolic bottleneck for increasing isoleucine production by C. glutamicum YILW.
2.2 Effect of pyc overexpression on isoleucine production Considering the short supply of oxaloacetate and downregulated pyc, it made sense that amplification of pyc was expected to increase oxaloacetate supply and consequently enhance isoleucine production. Considering the potential metabolic burden caused by plasmid and further engineering modification, pyc was overexpressed via replacement of its native promoter with a promoter of tuf gene, resulting in YILW-1, which has been proved to be a strong promoter in C. glutamicum
[16]. Transcription level of pyc was detected and found to be signif i cantly increased by 32.4 times in YILW-1.
Table4 Metabolic characterization of C. glutamicumstrains
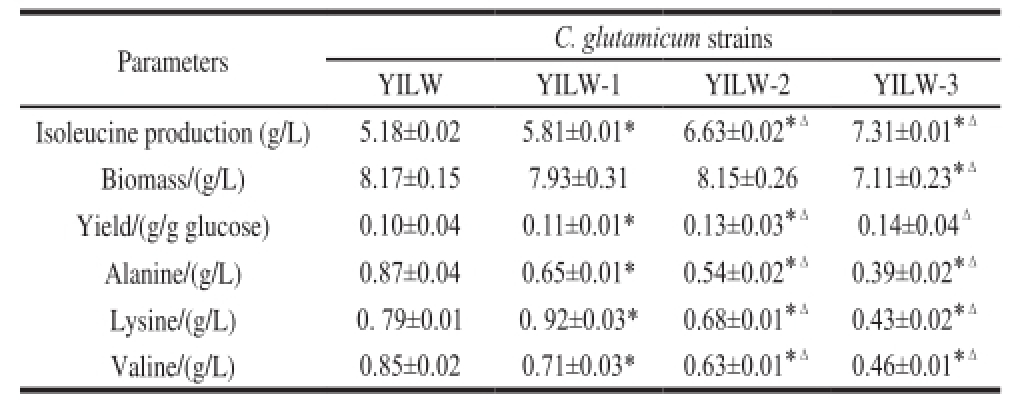
Note: *. Significantly different as compared with respective starting strains (P<0.05); Δ. Signif i cantly different as compared with those of YILW (P<0.05).
?
To assess the effects of pyc overexpression on isoleucine production, batch cultivations were carried out. As shown in Table 4, isoleucine production and yield by YILW-1 were 12.2% and 10.0% higher (elevated from 5.18 g/L and 0.10 g/g glucose to 5.81 g/L and 0.11 g/g glucose) as compared to those produced by C. glutamicum YILW, respectively. Intracellular oxaloacetate concentration was significant increased to 3.32 μmol/g in YILW-1(Table 3), demonstrating that a smaller oxaloacetate pool is indeed the metabolic bottleneck for increasing isoleucine production by C. glutamicum YILW.
The concentrations of common by-products during isoleucine fermentation were detected. It was found that alanine and valine accumulation decreased from 0.87 and 0.85 g/L to 0.65 and 0.71 g/L in YILW-1 (Table 4). Pyruvate is the precursor of both alanine and valine
[5], so overexpression of pyc may consume more pyruvate and consequently result in decreased alanine and valine accumulation. Interestingly, the content of lysine in YILW-1 was higher (0.92 g/L) than that in C. glutamicum YILW (0.79 g/L), which is possibly due to the reason that oxaloacetate is the precursor of both isoleucine and lysine and enhanced oxaloacetate supply increased the two products accumulation as a matter of course
[22]. Thus, it is necessary to drive more fl ux from oxaloacetate to isoleucine.
2.3 Effects of pyc and desensitized ilvBNC operon cooverexpression on isoleucine production by YILW-2
Higher lysine accumulation in YILW-1 indicated the necessity for enhancement of flux from oxaloacetate to isoleucine. Moreover, it should be notable that 2-ketobutyrate concentration was significantly higher (2.42 μmol/g) in YILW-1(Table 3).
In C. glutamicum, acetolactate synthase (AHAS) catalyzing 2-ketobutyrate to 2-aceto-2-hydroxybutyrate is encoded by ilvBN. The two genes form an operon together with ilvC gene and expression of the operon are controlled by transcriptional attenuation mediated by isoleucine
[23-25]. Transcription of ilvB was subsequently detected and was found to be 4.51-fold down-regulated in YILW-1. So it could be explained that accumulated 2-ketobutyrate was caused by attenuated transcription of ilvBNC operon by higher intracellular isoleucine concentration in YILW-1 (Table 3). Thus, defeating transcriptional attenuation of ilvBNC operon by isoleucine is urgent. The native promoter of ilvBN in YILW-1 was replaced by Ptac, which resulted in 15.1 times higher of ilvB transcription in YILW-2.
Batch cultivations were performed and results showed that the isoleucine production and yield by YILW-2 were 14.1% and 18.2% higher than those by YILW-1 (elevated from 5.81 and 0.11 g/g glucose to 6.63 and 0.13 g/g glucose, Table 4). Moreover, intracellular 2-ketobutyrate concentration was found to be significantly decreased to 1.52 μmol/g in YILW-2 (Table 3). The accumulations of alanine, lysine and valine accumulation by YILW-2 were decreased to 0.54, 0.68 and 0.63 g/L, which were 16.9%, 26.1% and 11.3% lower than those by YILW-1. It should be notable that ilvC in the operon was together overexpressed and its translation product, acetohydroxy acid isomeroreductase, needs NADPH as cofactor, so next work will focus on elevation of NADPH supply within cell.
Dihydroxy acid dehydratase (encoded by ilvD) and branched-chain amino acid aminotransferase (encoded by ilvE) are the other two non-rate-limiting enzymes forisoleucine synthesis. Although 2-oxo-3-methylvalerate produced by dihydroxy acid dehydratase is the direct precursor of isoleucine, we found that overexpression of ilvD in YILW-2 had no effect on promotion of isoleucine production (data not shown). Branched-chain amino acid aminotransferase (encoded by ilvE) catalyzes the last step of the pathway, while the reaction is nearly freely reversible (the equilibrium constant of the reaction was 0.5)
[25]. So it appeas that overexpression of ilvE makes little contribution to the increased isoleucine production.
2.4 Effect of enhancing isoleucine secretion on isoleucine production by YILW-3
As mentioned above, the last reaction for isoleucine synthesis catalyzed by branched-chain amino acid aminotransferase is almost freely reversible
[25]. So export of the synthetized isoleucine out of cell timely can decrease intracellular isoleucine concentration and thus promote the transaminase reaction. Furthermore, it is diff i cult to remove all the feedback controls in isoleucine synthesis pathway, so decreasing intracellular isoleucine concentration via enhancing its exportation can consequently decrease or defeat its feedback control.
Intracellular isoleucine concentration in C. glutamicum strains was found to be higher in YILW-2 (13.27 μmol/g) and YILW-1 (12.31 μmol/g) than in C. glutamicum YILW. So isoleucine secretion is probably the limiting step in YILW-2.
The isoleucine exporter is a two-component permease, encoded by brnE and brnF
[26]. Our early studies reported that overexpression of the two genes resulted in a significantly increased export rate and production of isoleucine
[13]. The plasmid carrying brnE and brnF was electroporated into YILW-2, obtaining YILW-3.
Batch cultivations were performed to assess the effects of brnE and brnF overexpression on isoleucine production. As shown in Table 4, isoleucine production and yield by YILW-3 were 10.3% and 7.7% higher (elevated from 6.63 and 0.13 g/g glucose to 7.31 and 0.14 g/g glucose) than those by YILW-2. Furthermore, accumulated levels of alanine, lysine and valine by YILW-3 were decreased to 0.39, 0.43 and 0.46 g/L, which was 27.8%, 36.8% and 27.0% lower than those by YILW-2. However, biomass of YILW-3 was decreased to 7.11 g/L, possibly resulting from metabolic burden caused by plasmid. So next work should be focused on overexpressing brnE and brnF in genome by replacing the promoters of brnE and brnF with strong promoter.
3 Conclusion
In this study, we took a strategy to enhance isoleucine production based on metabolite pool analysis and transcriptional analysis of key genes involved in isoleucine synthesis. Low intracellular oxaloacetate pool in C. glutamicum YILW was identif i ed as a potential metabolic bottleneck for isoleucine production. And overexpression of pyc and desensitized ilvBNC operon effectively increased oxaloacetate supply and enhanced the metabolic flux from oxaloacetate to isoleucine. Moreover, enhancing isoleucine secretion resulted in its production. The strategy led to 41.1% increase in isoleucine production (from 5.18 to 7.31 g/L) and 40.0% higher yield (from 0.10 to 0.14 g/g glucose) by YILW-3 as compared to those by C. glutamicum YILW. Strategy used in this study had potential applications for rational modif i cation of industrial microorganisms.
[1] IKEDA S, FUJITA I, YOSHINAGA F. Screening of L-isoleucine producers among ethionine resistant mutants of L-threonine producing bacteria[J]. Agricultural and Biological Chemistry, 1976, 40(3): 511-516. DOI:10.1271/bbb1961.40.511.
[2] PARK J H, LEE S Y. Fermentative production of branched chain amino acids: a focus on metabolic engineering[J]. Applied Microbiology Biotechnology, 2010, 85(3): 491-506. DOI:10.1007/s00253-009-2307-y.
[3] BLOMSTRAND E. A role for branched-chain amino acids in reducing central fatigue[J]. The Journal of Nutrition, 2006, 136(2): 544S-547S.
[4] ROSE W C, HANIES W J, JOHNSON J E. The role of the amino acids in human nutrition[J]. The Journal of Biological Chemistry, 1947, 146(2): 683-684.
[5] WANG J, WEN B, WANG J, et al. Enhancing L-isoleucine production by thrABC overexpression combined with alaT deletion in Corynebacterium glutamicum[J]. Applied Biochemistry and Biotechnology, 2013, 171(1): 20-30. DOI:10.1007/s12010-013-0321-0.
[6] SHIIO I, MIYAJIMA R. Concerted inhibition and its reversal by end products of aspartate kinase in Brevibacterium fl avum[J]. Journal of Biochemistry, 1969, 65(6): 849-859.
[7] YIN L H, HU X Q, XU D Q, et al. Co-expression of feedbackresistant threonine dehydratase and acetohydroxy acid synthase increase L-isoleucine production in Corynebacterium glutamicum[J]. Metabolic Engineering, 2012, 14(5): 542-550. DOI:10.1016/ j.ymben.2012.06.002.
[8] MIYAJIMA R, SHIIO I. Regulation of aspartate family amino acid biosynthesis in Brevibacterium fl avum: Ⅲ. properties of homoserine dehydrogenase[J]. Journal of Biochemistry, 1970, 68(3): 311-319.
[9] EIKMANNS B J, METZGER M, REINSCHEID D, et al. Amplif i cation of three threonine biosynthesis genes in Corynebacterium glutamicum and its influence on carbon flux in different strains[J]. Applied Microbiology and Biotechnology, 1991, 34(5): 617-622. DOI:10.1007/ BF00167910.
[10] EGGELING L, BOTT M. Handbook of Corynebacterium glutamicum[M]. Boca Raton: CRC Press, 2005: 520-521.
[11] BAILEY J E. Toward a science of metabolic engineering[J]. Science, 1991, 252: 1668-1675.
[12] ZHANG C L, DU S S, LIU Y, et al. Strategy for enhancing adenosine production under the guidance of transcriptional and metabolite pool analysis[J]. Biotechnology Letters, 2015, 37(7): 1361-1369. DOI:10.1007/s10529-015-1801-9.
[13] XIE X X, XU L L, SHI J M, et al. Effect of transport proteins on L-isoleucine production with the L-isoleucine-producing strain Corynebacterium glutamicum YILW[J]. Journal of Industrial Microbiology and Biotechnology, 2012, 39(10): 1549-1556. DOI:10.1007/s10295-012-1155-4.
[14] LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2
-ΔΔCTmethod[J]. Methods, 2001, 25(4): 402-408. DOI:10.1006/meth.2001.1262.
[15] BUCHHOLZ A, HURLEBAUS J, WANDREY C, et al. Metabolomics: quantification of intracellular metabolite dynamics[J]. Biomolecular Engineering, 2002, 19(1): 5-15. DOI:10.1016/S1389-0344(02)00003-5.
[16] BECKER J, KLOPPROGGE C, ZELDER O, et al. Amplified expression of fructose 1,6-bisphosphatase in Corynebacterium glutamicum increases in vivo fluxthrough the pentose phosphate pathway and lysine production on different carbon sources[J]. Applied Environmental Microbiology, 2005, 71(12): 8587-8596. DOI:10.1128/ AEM.71.12.8587-8596.2005.
[17] CHENG Y S, ZHOU Y J, YANG L, et al. Modification of histidine biosynthesis pathway genes and the impact on production of L-histidine in Corynebacterium glutamicum[J]. Biotechnology Letters, 2013, 35(5): 735-741. DOI:10.1007/s10529-013-1138-1.
[18] BECKER J, ZELDER O, HAFNER S, et al. From zero to hero-designbased systems metabolic engineering of Corynebacterium glutamicum for L-lysine production[J]. Metabolic Engineering, 2011, 13(2): 159-168. DOI:10.1016/j.ymben.2011.01.003.
[19] PETERS-WENDISCH P, STAMSEM K C, GOTKER S, et al. Biotin protein ligase from Corynebacterium glutamicum: role for growth and L-lysine production[J]. Applied Microbiology Biotechnology, 2012, 93(6): 2493-2502. DOI:10.1007/s00253-011-3771-8.
[20] SHIRAI T, FUJIMURA K, FURUSAWA C, et al. Study on roles of anaplerotic pathways in glutamate overproduction of Corynebacterium glutamicum by metabolic flux analysis[J]. Microbial Cell Factories, 2007, 6(19): 1-9. DOI:10.1186/1475-2859-6-19.
[21] SATO H, ORISHIMO K, SHIRAI T, et al. Distinct roles of two anaplerotic pathways in glutamate production induced by biotin limitation in Corynebacterium glutamicum[J]. Journal of Bioscience and Bioengineering, 2008, 106(1): 51-58. DOI:10.1263/jbb.106.51.
[22] PETERS-WENDISCH P G, EIKMANNS B J, THIERBACH G, et al. Phosphoenolpyruvate carboxylase in Corynebacterium glutamicum is dispensable for growth and lysine production[J]. FEMS Microbiology and Letters, 1993, 112(3): 269-274.
[23] MORBACH S, JUNGER C, SAHM H, et al. Attenuation control of ilvBNC in Corynebacterium glutamicum: evidence of leader peptide formation without the presence of a ribosome binding site[J]. Journal of Bioscience and Bioengineering, 2000, 90(5): 501-507. DOI:10.1016/S1389-1723(01)80030-X.
[24] ELISAKOVA V, PATEK M, HOLATKO J, et al. Feedbackresistant acetohydroxy acid synthase increases valine production in Corynebacterium glutamicum[J]. Applied and Environmental Microbiology, 2005, 71(1): 207-213. DOI:10.1128/AEM.71.1.207-213.2005.
[25] WILHELM C, EGGELING I, NASSENSTEIN A, et al. Limitations during hydroxybutyrate converison to isoleucine with Corynebacterium glutamicum, as analysed the formation of byproducts[J]. Applied Microbiology and Biotechnology, 1989, 31: 458-462. DOI:10.1007/ BF00270776.
[26] KENNERKNECHT N, SAHM H, YEN M R, et al. Export of L-isoleucine from Corynebacterium glutamicum: a two-gene-encoded member of a new translocator family[J]. Journal of Bacteriology, 2002, 184(14): 3947-3956. DOI:10.1128/JB.184.14.3947-3956.2002.
基于基因转录和代谢物分析的异亮氨酸生产菌谷氨酸棒状杆菌YILW的理性改造
温 冰,麻 杰,李智祥,张成林,徐庆阳,陈 宁*
(天津科技大学生物工程学院,代谢控制发酵技术国家地方联合工程实验室,天津 300457)
摘 要:为获得异亮氨酸生产菌谷氨酸棒状杆菌(Corynebacterium glutamicum)YILW的理性改造策略,考察该菌株与出发菌株C. glutamicum ATCC 13032异亮氨酸合成途径中关键酶及代谢产物的差异。结果表明,C. glutamicum YILW丙酮酸羧化酶编码基因pyc的下调表达使得其胞内草酰乙酸含量降低,过表达该基因显著增加胞内草酰乙酸含量及异亮氨酸产量(分别从1.32 μmol/g(细胞干质量,下同)和5.18 g/L提高至3.32 μmol/g和5.81 g/L),但副产物赖氨酸及胞内2-酮丁酸积累量提高。针对该问题采用强启动子替换手段过表达ilvBNC操纵子,使得其异亮氨酸产量提高至6.63 g/L。为进一步增加异亮氨酸合成,过表达输出载体编码基因brnE和brnF,其产量提高至7.31 g/L,较出发菌株C. glutamicum YILW提高41.1%,转化率提高40.0%。由此可见,在基因转录及代谢物分析结果指导下理性过表达pyc、ilvBNC操纵子及brnE和brnF能够显著提高异亮氨酸产量并降低副产物浓度。
关键词:谷氨酸棒杆菌;异亮氨酸;代谢库;草酰乙酸
中图分类号:Q935
文献标志码:A
文章编号:1002-6630(2017)04-0032-07
References:
收稿日期:2016-06-14
基金项目:国家高技术研究发展计划(863计划)项目(2013AA102106);国家自然科学基金青年科学基金项目(31300069);天津市科技特派员项目(15JCTPJC62800)
作者简介:温冰(1977—),女,高级工程师,博士,研究方向为氨基酸代谢控制发酵。E-mail:wbingr@126.com
引文格式:
DOI:10.7506/spkx1002-6630-201704006
*通信作者:陈宁(1963—),男,教授,博士,研究方向为氨基酸及其衍生物、核苷代谢工程和发酵工程。
E-mail:ningch@tust.edu.cn
WEN Bing, MA Jie, LI Zhixiang, et al. Rational design of Corynebacterium glutamicum YILW for isoleucine production based on gene transcription and metabolite analysis[J]. 食品科学, 2017, 38(4): 32-38.
DOI:10.7506/spkx1002-6630-201704006. http://www.spkx.net.cn
WEN Bing, MA Jie, LI Zhixiang, et al. Rational design of Corynebacterium glutamicum YILW for isoleucine production based on gene transcription and metabolite analysis[J]. Food Science, 2017, 38(4): 32-38. DOI:10.7506/spkx1002-6630-201704006. http://www.spkx.net.cn




 ±s (n=3). One-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test was used to determine significant difference, and the statistical signi fi cance was de fi ned as P≤0.05.
±s (n=3). One-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test was used to determine significant difference, and the statistical signi fi cance was de fi ned as P≤0.05.

