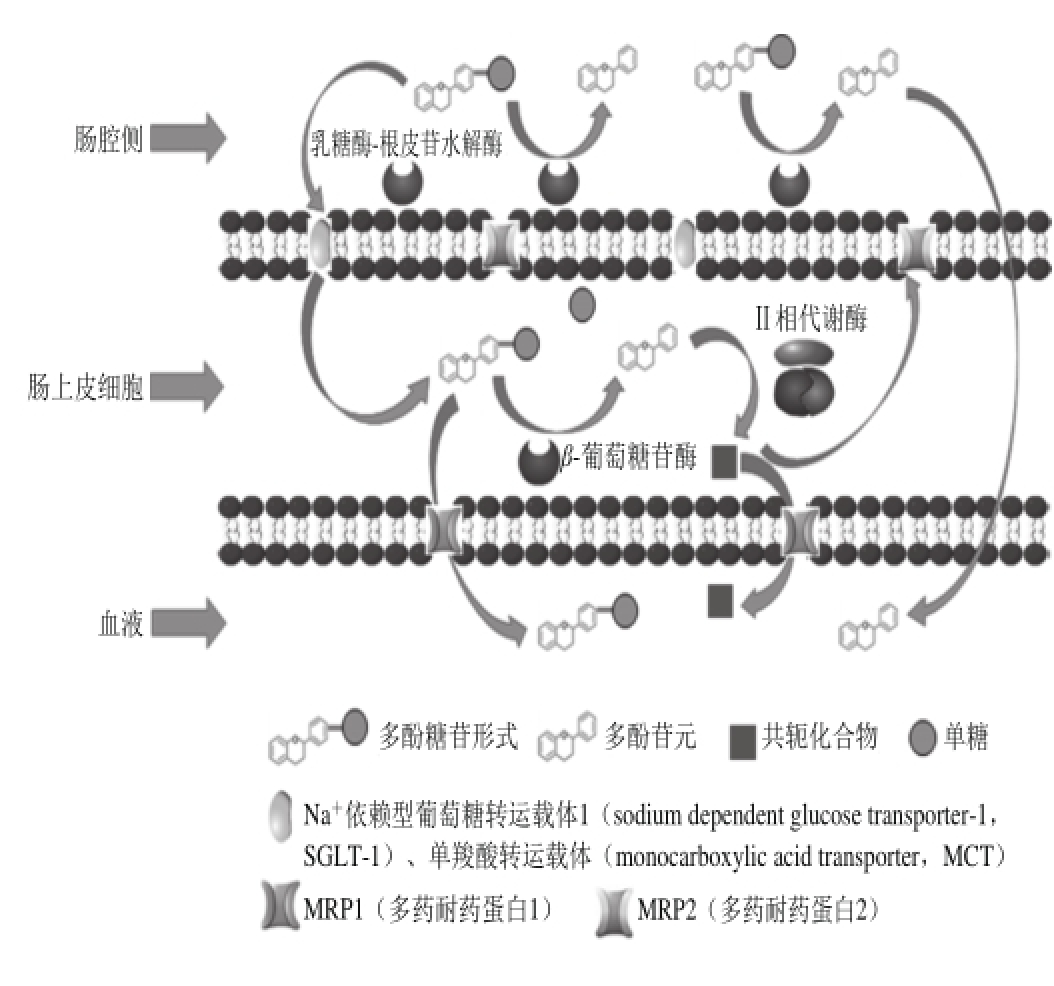
图1 膳食多酚在肠道中释放和吸收过程示意图[4]
Fig. 1 Schematic diagram for the process of intestinal release and absorption of dietary polyphenols[4]
左 丹1,廖 霞1,李 瑶1,石 芳1,王丽颖1,明 建1,2,*
(1.西南大学食品科学学院,重庆 400715;2.重庆市特色食品工程技术研究中心,重庆 400715)
摘 要:膳食多酚是植物性食物的重要成分,不仅与食品的风味和颜色等品质直接相关,还有利于人体健康。由于它们在基质释放、消化、吸收、代谢和组织分布期间发生改变,使得各种酚类化合物生物利用度有一定的差异。饮食中含量最丰富的不一定具有最佳生物利用度,多酚的吸收转化及生物利用度是多酚生物活性主要研究热点之一。本文以多酚肠道吸收机制为基点,综述了膳食多酚在胃肠道释放、吸收、代谢等过程,以期为多酚类保健食品的研发提供依据。
关键词:膳食多酚;生物利用度;吸收;代谢;转运
多酚是植物体内复杂酚类次生代谢产物,由一个或多个芳环化合物与一个或多个羟基组成,主要包括酚酸、类黄酮、对二苯代乙烯、香豆素、单宁等。具有抗氧化、抗癌、降血压、降血糖、抗菌等多方面保健和药理作用[1]。膳食多酚主要来源于水果、蔬菜和饮料,每100 g新鲜果蔬中含有200~300 mg多酚类化合物[2]。成年人通过膳食摄入多酚类化合物的总量约为(1 193±510) mg/d[3]。
尽管多酚在膳食中含量很高,但它们在体内的生物利用度较差。由于膳食多酚多以糖基化或酯类、聚合物形式存在于植物性食物中,吸收前需经过肠道酶(如乳糖酶-根皮苷水解酶(lactase-phlorizin hydrolase,LPH))或微生物菌群水解,然后在细胞色素P450家族(cytochromes P450,CYPs)、儿茶酚-O-甲基转移酶(catechol-O-methyltransferase,COMT)、硫酸基转移酶(sulfotransferase,SULTs)、葡萄糖醛酸转移酶(UDP-glucuronosyltransferases,UGTs)等代谢酶作用下进行广泛代谢,改变其原本的结构,之后一部分多酚进入血液循环,一部分被肠道外排转运体如P-糖蛋白(P-glycoprotein,P-gp)、多药耐药相关蛋白2(multidrug resistance-associated protein 2,MRP2)、乳腺癌耐药蛋白(breast cancer resistance protein,BCRP)等外排回肠腔,导致生物利用度降低(图1)。

图1 膳食多酚在肠道中释放和吸收过程示意图[4]
Fig. 1 Schematic diagram for the process of intestinal release and absorption of dietary polyphenols[4]
本文以多酚肠道吸收机制为基点,综述膳食多酚在胃肠道释放、吸收、代谢等体内过程,以期为多酚类保健食品的研发提供依据。
多酚在胃肠消化期间从食物基质中逐渐完全释放出来,生物有效性增加,可用于吸收或在胃肠道中发挥其生物学效应[5]。释放水平主要由多酚结构和肠道菌群的差异决定。
1.1 胃中释放
大多数多酚在胃消化期间被释放,主要由胃消化酶介导。如低聚合单宁(单体,二聚体和三聚体)与唾液蛋白形成的复合物可以通过胃消化被破坏,导致单宁释放,相反单宁四聚体和五聚体形成的复合物耐胃消化更显著[6]。如苹果中65%的多酚和黄酮类化合物在胃中释放,小肠中释放量小于10%[7]。
酸性条件下,一些多酚可以在唾液亚硝酸盐作用下在胃中被释放。如槲皮素、山奈酚、花青素、儿茶素类、咖啡酸、绿原酸、3,4,5-三羟基苯甲酸等均能被亚硝酸盐氧化[8-9]。Hirota等[10]发现亚硝酸盐能与苹果甲醇提取物在酸性缓冲溶液(pH 2.0)和酸化唾液中反应,反应速率依次为绿原酸<(-)-表儿茶素<原花青素B2。
1.2 小肠中释放
一些多酚在小肠温和碱性条件下高度敏感,且在弱碱性条件下胰腺和胆汁分泌的一些酶(如淀粉酶、胰蛋白酶、胰凝乳蛋白酶原等)被激活,使这些多酚能以良好的比例转化为具有不同化学性质的其他未知结构形式,从而具有不同的生物利用度和生物活性[11]。Bermúdez-Soto等[12]研究野樱桃汁酚类成分时发现胃消化对其酚类成分(花青素、黄烷-3-醇、黄酮醇和咖啡酸衍生物)没有实质性影响,而在胰酶消化过程中有明显改变。用胰酶处理2 h约释放43%,黄酮醇和黄烷-3-醇含量分别下降26%和19%,新绿原酸含量下降28%,绿原酸含量提高24%。此外,位于小肠黏膜上皮细胞刷状缘上的糖苷酶如LPH能催化糖基释放苷元 。
1.3 结肠中释放
膳食中不可萃取多酚在口腔、胃、小肠作用下不能从食品基质中被释放,到达结肠后,在肠道菌群酶作用下糖苷键断裂形成苷元或进一步发生环裂解产生酚酸,被吸收或排泄[13]。释放机制有两种,一是与糖、有机酸、脂类等酯化的酚类在结肠菌群产生的酯酶作用下断裂酯键。二是鞣花单宁等聚合物则需微生物裂解其共轭基团。据估计,48%的膳食多酚在小肠变得生物可接受,其余52%在大肠[14]。
只有少量的肠道微生物(如大肠杆菌、双歧杆菌、乳杆菌、真杆菌等)能将膳食多酚进行生物转化[15]。产物主要是苯乙酸和苯丙酸或其羟基化合物,酚酸经肠道直接吸收或与肠道细胞表面特定或非特定受体结合,通过影响多个基因的表达和信号转导发挥生理功能[16]。Kubow等[17]研究发现紫肉甘薯中花色素苷降解和生物有效性依赖于结肠微生物的分解。Padayachee等[18]研究发现果蔬中与胞壁结合的多酚在结肠中通过细胞壁降解菌作用而被释放。
1.4 影响多酚释放的主要因素
胃肠道pH值。多酚在消化过程中降解和活性下降主要是由pH值变化引起的[19]。如儿茶素在模拟酸性胃环境中较稳定,在近中性或碱性环境下大幅度降解,儿茶素类衍生物的降解大多发生在小肠环境,在肠液环境的损失量通常比胃环境高10~20 倍[20]。胃消化过程中总酚、黄酮类和花青素类的生物可及性增加,而在肠道环境中酚酸和白藜芦醇降解,儿茶素、槲皮素则相对较稳定[21]。
与其他营养物质相互作用。膳食多酚能与食品基质的某些成分,如蛋白质、铁、膳食纤维相互作用,形成复合物,变得不可吸收,影响其生物有效性,但这种相互作用能被消化酶或微生物破坏[22]。Mosele等[23]研究发现果胶的存在会阻碍酚类化合物的溶解,肠消化过程中果胶的降解有利于酚类化合物的释放。
2.1 吸收部位
膳食多酚摄入后,一部分先在胃部被低程度吸收,如黄酮醇(槲皮素)、异黄酮苷元(大豆素和染料木素)、花色苷、酚酸(阿魏酸、咖啡酸、绿原酸、没食子酸),主要是单酚酸类物质[24]。只有少部分多酚(5%~10%)在小肠吸收(主要是游离态多酚),其余未被吸收多酚(主要是结合态多酚)到达结肠,在肠中微生物菌群作用下进行分解、释放、吸收[25]。
2.2 吸收机制
影响膳食多酚肠道吸收机制的因素很多,如烷基链长、羟基取代、化学结构、分子质量等。小分子多酚如没食子酸、异黄酮、儿茶素等很容易通过肠道吸收,大分子多酚如花青素吸收较差[26]。肠道主要通过主动转运和被动扩散两种机制来吸收多酚,以被动扩散为主。
2.2.1 主动运输
主动运输是指物质逆浓度梯度运输,由细胞膜上转运蛋白介导。与相应苷元相比,酚苷类物质亲水性较好,很难通过被动扩散跨过肠上皮细胞膜,主要通过主动运输[27]。主要包括Na+依赖的主动转运和MCT介导的主动转运两种方式。糖苷被载体运输到上皮细胞内,由细胞内β-葡萄糖苷酶(cytosolic β-glucosidase,CBG)介导其水解。大量研究证实,SGLT-1参与肉桂酸、绿原酸、阿魏酸、咖啡酸、槲皮素-4-葡萄糖苷、大黄素和白藜芦醇等转运过程[28]。此外,易化性葡萄糖转运载体-2(facilitated glucose transporter-2,GLUT-2)也参与一些膳食多酚的转运[29]。李素云[30]研究表明SGLT-1是槲皮素及其两个糖苷(槲皮苷和异槲皮苷)在肠道吸收中的转运蛋白之一,同时槲皮苷也通过GLUT-2转运吸收。
MCT的底物是含有阴离子羧酸基团、非极性侧链或芳香族疏水性基团的酚类物质,如咖啡酸、阿魏酸、表儿茶素没食子酸,主要为单羧酸类物质。Dang Yunjie等[31]研究证实MCT参与了菠萝叶提取物(extract of Ananas comosus L. leaves,EAL)中p-香豆酸和咖啡酸的跨膜转运,两种物质的渗透具有浓度和时间依赖性。关于哪些MCT参与多酚的转运仍未明确,除已报道的MCT3、MCT4、MCT5、MCT6外,还有一些多酚的相关转运蛋白[4]。
2.2.2 被动扩散
多酚被消化后释放的苷元主要通过被动扩散进入肠上皮细胞。被动扩散是由浓度梯度驱使,有跨细胞途径(通过肠上皮细胞膜进入血液)或者细胞旁路途径(通过肠上皮细胞间的紧密联接进入血液)两种途径,取决于物质的疏水性。含羟基较多的多酚亲水性好,它们会通过细胞旁路途径被优先吸收,如(+)-儿茶素、(-)-表儿茶素、原花青素B2等[32]。山楂树叶和花中原花色素可通过跨细胞途径和细胞旁路途径被吸收,原花青素B2和聚合度4~6的原花青素渗透率都很低,分子质量越大渗透性越差[33],Rastogi等[34]研究发现6 种酚类化合物(白杨素、咖啡酸、没食子酸、槲皮素、白藜芦醇、芦丁)通过Caco-2细胞吸收低且在人工膜渗透性(parallel artif i cial membrane permeability assay,PAMPA)差,其跨膜运输主要是通过被动扩散。Kimura等[35]也证实川陈皮素(nobiletin,NBL)在Caco-2细胞顶膜的吸收主要是由被动扩散过程介导。
此外,两种方式也可同时参与膳食多酚的吸收。如白藜芦醇被血管内皮细胞吸收是由被动扩散和SGLT-1介导的过程[36]。Konishi[37]研究发现槲皮素3种代谢产物中,3,4-二羟基苯乙酸(3,4-dihydroxyphenylacetic acid,DHPA)通过细胞旁路途径运输,4-羟基-3-甲氧基苯基乙酸(4-hydroxy-3-methoxyphenylacetic acid,HMPA)和间-羟基苯乙酸(m-hydroxyphenylacetic acid,mHPA)的吸收由MCT介导。
膳食多酚吸收后进入血液循环之前,会受到Ⅰ相和Ⅱ相代谢酶作用,一部分进入血液循环,另一部分被外排转运体泵回肠腔。大量研究表明,许多代谢物比它们的前体具有更活跃的生物功能[38]。
3.1 Ⅰ相代谢
Ⅰ相代谢反应有氧化反应、还原反应和水解反应,反应使分子结构中引入或暴露出极性基团,如产生羟基、羧基、巯基、氨基等。多种酶参与Ⅰ相代谢反应,包括还原酶、酯酶、CYPs等。CYPs是人体内最重要的Ⅰ相代谢酶系,其中CYP1、CYP2、CYP3家族负责外源性物质的代谢。在肠吸收中,50%以上药物的Ⅰ相代谢与CYP3A4相关[39]。
作为一种单加氧酶,CYPs可参与多种催化氧化反应,引入极性基团(如羟基),增加物质亲水性,有助于Ⅱ相结合酶的利用或排泄[40]。如染料木黄酮和NBL容易被CYPs氧化[41]。此外,膳食多酚可与CYPs相互作用或影响CYPs基因表达而改变CYPs活性[42]。
3.2 Ⅱ相代谢
Ⅱ相代谢是结合反应,通常是药物或Ⅰ相反应生成的代谢产物结构中的极性官能团(如羟基、氨基、硝基和羧基等)与机体内源性物质发生偶联或结合生成各种结合物的过程。由于膳食多酚类结构使他们不适于细胞色素P450基板,大多数膳食多酚不进行Ⅰ相代谢,可直接进行Ⅱ相代谢,主要包括甲基化、硫酸盐化和葡糖醛酸化,并通过增加其亲水性促进在胆汁和尿中消除[43]。Ⅱ相代谢发生部位主要是肠和肝。del Rio等[44]报道浆果中酚类化合物的吸收形式是以葡萄糖苷、硫酸酯和甲基化产物从胃肠道进入循环系统。
甲基化反应由COMT介导,它能催化S-腺苷-L-甲硫氨酸的甲基转移至有邻苯二酚结构的多酚,如槲皮素、儿茶素、咖啡酸、木犀草素、花青素等。反应主要发生在多酚的C3’位,但也有一小部分形成4’-O-甲基化产物[45]。SULTs催化硫酸基团从3’-磷酸腺苷-5’-磷酸硫酸转移到多酚的C3’、C4’、C5、C7位羟基上[46]。UGTs催化葡萄糖醛酸从UDP-葡萄糖醛酸转移至多酚的C7位、C4’位羟基上,C7位最易发生,而C5位几乎不发生葡萄糖醛酸化[47]。Chen Jun等[48]研究6 种异黄酮(染料木黄酮、黄豆苷元、黄豆黄素、芒柄花素、鹰嘴豆素A和樱黄素)在Caco-2细胞单层上的吸收代谢,发现樱黄素主要被硫酸盐化,另外5 种异黄酮被葡萄糖醛酸化,C7是葡萄糖醛酸化的主要位点,C4’是硫酸盐化的唯一位点。
3 种类型变化形式的重要性取决于底物性质和摄入剂量,化合物结构中细小差别将对其代谢通路产生显著性影响[49]。如大鼠静脉注射多酚剂量增加时,硫酸盐化作用明显转变为葡萄醛酸化[50]。腾增辉[51]研究发现芹菜素、白藜芦醇、大黄酚和大黄素在肠道Ⅱ相代谢酶的作用下,易生成葡萄糖醛酸化和硫酸酯化代谢物,Caco-2细胞中大黄素和大黄酚与肠道UGTs的亲和力更高,容易生成葡萄糖醛酸化代谢产物,远多于硫酸酯化的代谢产物;而Caco-2细胞中芹菜素和白藜芦醇对SULT亦具有较高的亲和力,葡萄糖醛酸化与硫酸酯化代谢产物之间的量差别不大。
广泛分布于肠上皮细胞黏膜的肠道外排转运体可以将吸收后的部分多酚泵出细胞膜外,也可与代谢酶发生协同作用,阻止膳食多酚的吸收,这是多酚生物利用度较低的重要原因。主要有3 种ATP-依赖性外排转运体(ATP-binding cassette (ABC) transporter),即P-gp、MRP2、BCRP,它们能依赖ATP分解释放的能量将底物外排回肠腔。大部分P-gp底物是结构和药理活性不相关的疏水、亲脂性、中性或弱碱性化合物[52]。MRP2主要是有机阴离子的载体如谷胱甘肽结合物、葡萄糖醛酸结合物、硫酸结合物等。不同的是,MRP1、MRP3、MRP4、MRP5等能将多酚运输至血液,对多酚的吸收有促进作用。BCRP属于半ABC转运体,要形成同型二聚体后才可发挥转运的功能。
3 种外排转运体的底物不完全相同但有交叉。有研究发现转运体P-gp对灯盏花素吸收基本无影响,MRP2可将吸收的灯盏花素从肠上皮细胞内又转运回肠腔,从而降低其吸收[53]。何卉等[54]研究发现白藜芦醇及白藜芦醇苷具有抑制P-gp外排的作用,但其本身不被P-gp外排,而是被MRP2外排。王婷婷等[55]研究发现P-gp与BCRP外排作用是田蓟苷小肠吸收的主要外排机制,能够依赖SGLT-1实现在小肠的吸收转运。
最后剩余的多酚代谢衍生物通过门静脉进入血液到达肝脏发生更多Ⅱ相代谢,其中部分代谢物通过胆汁分泌系统再次进入肠道,经肠道微生物酶代谢后重新被吸收,其余的共轭代谢物再次进入血液分布到其他组织,最终通过尿或粪便排泄[56]。
大多数膳食多酚以糖基化或酯类、聚合物形式存在于植物性食物中,必须经过肠道酶或微生物菌群水解,释放酚苷元才能被吸收。在吸收过程中,膳食多酚先后在肠和肝脏中代谢,主要包括甲基化、硫酸化、葡萄糖醛酸化。吸收后一部分多酚进入血液循环,一部分被外排回肠腔。酚类化合物的母核结构以及羟基的数目和位置影响它们与代谢酶以及药物转运载体的亲和力,在胃肠道体现不同的吸收特性,最终影响膳食多酚的生理效应和功能活性。
多酚有效成分在体内生物效应多表现为高代谢和弱生物利用度,这是多酚作为药物或保健食品开发应用的障碍和限制。未来在以下几点需要有更全面的研究。一是多酚体内药动学过程,许多膳食多酚的生物转化和生物利用度仍不清楚,如关于膳食多酚在人体内胃肠道吸收、组织分布和生物转化途径等关键问题的数据需要被确定;二是膳食多酚代谢物的生物活性需要科学评价;三是如何提高多酚的生物利用度。
参考文献:
[1] ANCILLOTTI C, CIOFI L, PUCCI D, et al. Polyphenolic prof i les and antioxidant and antiradical activity of Italian berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. subsp. gaultherioides (Bigelow) S.B. Young[J]. Food Chemistry, 2016, 204: 176-184. DOI:10.1016/j.foodchem.2016.02.106.
[2] SCALBERT A, MANACH C, MORAND C, et al. Dietary polyphenols and the prevention of diseases[J]. Critical Reviews in Food Science and Nutrition, 2005, 45(4): 287-306. DOI:10.1080/1040869059096.
[3] PÉREZ-JIMÉNEZ J, FEZEU L, TOUVIER M, et al. Dietary intake of 337 polyphenols in French adults[J]. American Journal of Clinical Nutrition, 2011, 93(6): 1220-1228. DOI:10.3945/ajcn.110.007096.
[4] BOHN T. Dietary factors affecting polyphenol bioavailability[J]. Nutrition Reviews, 2014, 72(7): 429-452. DOI:10.1111/nure.12114.
[5] SANZ-BUENHOMBRE M, VILLANUEVA S, MORO C, et al. Bioavailability and the mechanism of action of a grape extract rich in polyphenols in cholesterol homeostasis[J]. Journal of Functional Foods, 2016, 21: 178-185. DOI:10.1016/j.jff.2015.11.044.
[6] SOARES S, BRANDÃO E, MATEUS N, et al. Interaction between red wine procyanidins and salivary proteins: effect of stomach digestion on the resulting complexes[J]. RSC Advances, 2015, 5(17): 12664-12670. DOI:10.1039/c4ra13403f.
[7] BOUAYED J, HOFFMANN L, BOHN T. Total phenolics, fl avonoids, anthocyanins and antioxidant activity following simulated gastrointestinal digestion and dialysis of apple varieties: bioaccessibility and potential uptake[J]. Food Chemistry, 2011, 128(1): 14-21. DOI:10.1016/j.foodchem.2011.02.052.
[8] GAGO B, LUNDBERG J O, BARBOSA R M, et al. Red winedependent reduction of nitrite to nitric oxide in the stomach[J]. Free Radical Biology and Medicine, 2007, 43(9): 1233-1242. DOI:10.1016/ j.freeradbiomed.2007.06.007.
[9] TAKAHAMA U, YAMAUCHI R, HIROTA S. Reactions of (+)-catechin with salivary nitrite and thiocyanate under conditions simulating the gastric lumen: production of dinitrosocatechin and its thiocyanate conjugate[J]. Free Radical Research, 2014, 48(8): 965-966. DOI:10.3109/10715762.2014.929121.
[10] HIROTA S, TAKAHAMA U. Reactions of apple fruit polyphenols with nitrite under conditions of the gastric lumen: generation of nitric oxide and formation of nitrosocatechins[J]. Food Science and Technology Research, 2014, 20(2): 439-447. DOI:10.3136/fstr.20.439.
[11] VELDERRAIN-RODRÍGUEZ G R, PALAFOX-CARLOS H, WALLMEDRANO A, et al. Phenolic compounds: their journey after intake[J]. Food and Function, 2014, 5(2): 189-197. DOI:10.1039/c3fo60361j.
[12] BERMÚDEZ-SOTO M J, TOMÁS-BARBERÁN F A, GARCÍACONESA M T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion[J]. Food Chemistry, 2007, 102(3): 865-874. DOI:10.1016/ j.foodchem.2006.06.025.
[13] SERRA A, MACIÀ A, ROMERO M P, et al. Metabolic pathways of the colonic metabolism of fl avonoids (f l avonols, fl avones and fl avanones) and phenolic acids[J]. Food Chemistry, 2012, 130(2): 383-393. DOI:10.1016/j.foodchem.2011.07.055.
[14] SAURA-CALIXTO F, SERRANO J, GOÑI I. Intake and bioaccessibility of total polyphenols in a whole diet[J]. Food Chemistry, 2007, 101(2): 492-501. DOI:10.1016/j.foodchem.2006.02.006.
[15] CARDONA F, ANDRÉS-LACUEVA C, TULIPANI S, et al. Benef i ts of polyphenols on gut microbiota and implications in human health[J]. Journal of Nutritional Biochemistry, 2013, 24(8): 1415-1422. DOI:10.1016/j.jnutbio.2013.05.001.
[16] QIN B, DAWSON H D, SCHOENE N W, et al. Cinnamon polyphenols regulate multiple metabolic pathways involved in insulin signaling and intestinal lipoprotein metabolism of small intestinal enterocytes[J]. Nutrition, 2012, 28(11/12): 1172-1179. DOI:10.1016/ j.nu t.2012.03.020.
[17] KUBOW S, ISKANDAR M M, SABALLY K, et al. Biotransformation of anthocyanins from two purple-fleshed sweet potato accessions in a dynamic gastrointestinal system[J]. Food Chemistry, 2016, 192: 171-177. DOI:10.1016/j.foodchem.2015.06.105.
[18] PADAYACHEE A, NETZEL G, NETZEL M, et al. Lack of release of bound anthocyanins and phenolic acids from carrot plant cell walls and model composites during simulated gastric and small intestinal digestion[J]. Food and Function, 2013, 4(6): 906-916. DOI:10.1039/ c3fo60091b.
[19] ZHANG Q, RUI X, LI W, et al. Anti-swarming and -biof i lm activities of rose phenolic extract during simulated in vitro gastrointestinal digestion[J]. Food Control, 2016, 64: 189-195. DOI:10.1016/ j.foodcont.2015.12.030.
[20] NEILSON A P, HOPF A S, COOPER B R, et al. Catechin degradation with concurrent formation of homo- and heterocatechin dimers during in vitro digestion[J]. Journal of Agricultural and Food Chemistry, 2007, 55(22): 8941-8949. DOI:10.1021/jf071645m.
[21] TAGLIAZUCCHI D, VERZELLONI E, BERTOLINI D, et al. In vitro bio-accessibility and antioxidant activity of grape polyphenols[J]. Food Chemistry, 2010, 120(2): 599-606. DOI:10.1016/ j.foodchem.2009.10.030.
[22] ACOSTA-ESTRADA B A, GUTIÉRREZ-URIBE J A, SERNASALDÍVAR S O. Bound phenolics in foods, a review[J]. Food Chemistry, 2014, 152: 46-55. DOI:10.1016/j.foodchem.2013.11.093.
[23] MOSELE J I, MACIÀ A, ROMERO M P, et al. Stability and metabolism of arbutus unedo bioactive compounds (phenolics and antioxidants) under in vitro digestion and colonic fermentation[J]. Food Chemistry, 2016, 201: 120-130. DOI:10.1016/j.foodchem.2016.01.076.
[24] D’ARCHIVIO M, FILESI C, DI B R, et al. Polyphenols, dietary sources and bioavailability[J]. Annali Dellistituto Superiore Di Sanità, 2007, 43(4): 348-361.
[25] MOSELE J I, MACIÀ A, ROMERO M P, et al. Application of in vitro gastrointestinal digestion and colonic fermentation models to pomegranate products (juice, pulp and peel extract) to study the stability and catabolism of phenolic compounds[J]. Journal of Functional Foods, 2015, 14: 529-540. DOI:10.1016/j.jff.2015.02.026.
[26] MARTIN K R, APPEL C L. Polyphenols as dietary supplements: a double-edged sword[J]. Nutrition and Dietary Supplements, 2010, 2: 1-12. [27] HE J, GIUSTI M M. Anthocyanins: natural colorants with healthpromoting properties[J]. Annual Review of Food Science and Technology, 2010, 1: 163-187. DOI:10.1146/annurev.food.080708.100754.
[28] REQUENA T, MONAGAS M, POZO-BAYÓN M A, et al. Perspectives of the potential implications of wine polyphenols on human oral and gut microbiota[J]. Trends in Food Science and Technology, 2010, 21(7): 332-344. DOI:10.1016/j.tifs.2010.04.004.
[29] MANZANO S, WILLIAMSON G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells[J]. Molecular Nutrition and Food Research, 2010, 54(12): 1773-1780. DOI:10.1002/mnfr.201000019.
[30] 李素云. 槲皮素及其糖苷在Caco-2细胞模型上的吸收和代谢研究[D].北京: 中国人民解放军军事医学科学院, 2010: 45-52.
[31] DANG Y J, ZHU C Y. Genomic study of the absorption mechanism of p-coumaric acid and caffeic acid of extract of Ananas Comosus L. leaves[J]. Journal of Food Science, 2015, 80(3): 504-509. DOI:10.1111/1750-3841.12774.
[32] KOSIŃSKA A, ANDLAUER W. Cocoa polyphenols are absorbed in Caco-2 cell model of intestinal epithelium[J]. Food Chemistry, 2012, 135(3): 999-1005. DOI:10.1016/j.foodchem.2012.05.101.
[33] ZUMDICK S, DETERS A, HENSEL A. In vitro intestinal transport of oligomeric procyanidins (DP 2 to 4) across monolayers of Caco-2 cells[J]. Fitoterapia, 2012, 83(7): 1210-1217. DOI:10.1016/j.f i tote.2012.06.013.
[34] RASTOGI H, JANA S. Evaluation of physicochemical properties and intestinal permeability of six dietary polyphenols in human intestinal colon adenocarcinoma Caco-2 cells[J]. European Journal of Drug Metabolism and Pharmacokinetics, 2016, 41(1): 33-43. DOI:10.1007/ s13318-014-0234-5.
[35] KIMURA O, OHTA C, KOGA N, et al. Carrier-mediated uptake of nobiletin, a citrus polymethoxy flavonoid, in human intestinal Caco-2 cells[J]. Food Chemistry, 2014, 154: 145-150. DOI:10.1016/foodchem.2013.12.069.
[36] CHEN M L, YI L, JIN X, et al. Absorption of resveratrol by vascular endothelial cells through passive diffusion and an SGLT1-mediated pathway[J]. Journal of Nutritional Biochemistry, 2013, 24(11): 1823-1829. DOI:10.1016/j.jnutbio.2013.04.003.
[37] KONISHI Y. Transepithelial transport of microbial metabolites of quercetin in intestinal Caco-2 cell monolayers[J]. Journal of Agricultural and Food Chemistry, 2005, 53(3): 601-607. DOI:10.1021/jf0486621.
[38] CHIOU Y S, WU J C, HUANG Q, et al. Metabolic and colonic microbiota transformation may enhance the bioactivities of dietary polyphenols[J]. Journal of Functional Foods, 2014, 7: 3-25. DOI:10.1016/j.jff.2013.08.006.
[39] YANG J, TUCKER G T, ROSTAMI-HODJEGAN A. Cytochrome P450 3A expression and activity in the human small intestine[J]. Clinical Pharmacology and Therapeutics, 2004, 76(4): 391-391. DOI:10.1016/j.clpt.2004.07.001.
[40] KIM S B, CHO S S, CHO H J, et al. Modulation of hepatic cytochrome P450 enzymes by curcumin and its pharmacokinetic consequences in sprague-dawley rats[J]. Pharmacognosy Magazine, 2015, 11(44): 580-584. DOI:10.4103/0973-1296.172965.
[41] KOGA N, OHTA C, KATO Y, et al. In vitro metabolism of nobiletin, a polymethoxy-f l avonoid, by human liver microsomes and cytochrome P450[J]. Xenobiotica, 2011, 41(11): 927-933. DOI:10.3109/00498254. 2011.593208.
[42] BASHEER L, KEREM Z. Interactions between CYP3A4 and dietary polyphenols[J]. Oxidative Medicine and Cellular Longevity, 2015: 1-15. DOI:10.1155/2015/854015.
[43] BOHN T, MCDOUGALL G J, ALEGRÍA A, et al. Mind the gapdef i cits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites position paper focusing on carotenoids and polyphenols[J]. Molecular Nutrition and Food Research, 2015, 59(7): 1307-1323. DOI:10.1002/mnfr.201400745.
[44] del RIO D, BORGES G, CROZIER A. Berry fl avonoids and phenolics: bioavailability and evidence of protective effects[J]. British Journal of Nutrition, 2010, 104(3): 67-90. DOI:10.1017/S0007114510003958.
[45] LEE M J, MALIAKAL P L, CHEN L, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability[J]. Cancer Epidemiology Biomarkers and Prevention, 2002, 11: 1025-1032.
[46] JAMES M O, AMBADAPADI S. Interactions of cytosolic sulfotransferases with xenobiotics[J]. Drug Metabolism Reviews, 2013, 45(4): 401-414. DOI:10.3109/03602532.2013.835613.
[47] TANG L, FENG Q, ZHAO J, et al. Involvement of UDP-glucuronosyltranferases and sulfotransferases in the liver and intestinal first-pass metabolism of seven flavones in C57 mice and humans in vitro[J]. Food and Chemical Toxicology, 2012, 50(5): 1460-1467. DOI:10.1016/j.fct.2012.01.018.
[48] CHEN J, LIN H M, HU M. Absorption and metabolism of genistein and its fi ve isof l avone analogs in the human intestinal Caco-2 model[J]. Cancer Chemotherapy and Pharmacology, 2005, 55(2): 159-169. DOI:10.1007/s00280-004-0842-x.
[49] PANDAREESH M D, MYTHRI R B, BHARATH M M S. Bioavailability of dietary polyphenols: factors contributing to their clinical application in CNS diseases[J]. Neurochemistry International, 2015, 89: 198-208. DOI:10.1016/j.neuint.2015.07.003.
[50] KOSTER H, HALSEMA I, SCHOLTENS E, et al. Dose-dependent shifts in the sulfation and glucuronidation of phenolic compounds in the rat in vivo and in isolated hepatocytes: the role of saturation of phenol sulfotransferase[J]. Biochemical Pharmacology, 1981, 30(18): 2569-2575. DOI:10.1016/0006-2952(81)90584-0.
[51] 滕增辉. 天然多酚类化合物的肠道转运与代谢研究[D]. 西安: 第四军医大学, 2007: 91-110.
[52] LIU Z H, LIU K X. The transporters of intestinal tract and their study methods[J]. Acta Pharmaceutica Sinica, 2011, 46(4): 370-376.
[53] 张海燕, 平其能. 药物转运蛋白对灯盏花素小肠吸收的影响[J]. 中国药科大学学报, 2007, 38(1): 60-64. DOI:10.3321/ j.issn:1000-5048.2007.01.015.
[54] 何卉, 陈西敬, 王广基. 白藜芦醇及其糖苷白藜芦醇苷与药物外排转运体的相互作用研究[J]. 中国临床药理学与治疗学, 2008, 13(4): 366-372.
[55] 王婷婷, 李伟, 袁勇, 等. 大鼠单向灌流模型研究田蓟苷的在体肠吸收[J]. 中国中药杂志, 2013, 38(7): 1079-1082. DOI:10.4268/ cjcmm20130731.
[56] HOLLMAN P C H, CASSIDY A, COMTE B, et al. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established[J]. The Journal of Nutrition, 2011, 141(5): 989-1009. DOI:10.3945/jn.110.131490.
Progress in Research on Dietary Polyphenols Metabolism Based on a Mechanism Involving Intestinal Absorption
ZUO Dan1, LIAO Xia1, LI Yao1, SHI Fang1, WANG Liying1, MING Jian1,2,*
(1. College of Food Science, Southwest University, Chongqing 400715, China; 2. Chongqing Engineering Research Center of Regional Food, Chongqing 400715, China)
Abstract:Dietary polyphenols, an important class of components in plant-derived foods, is not only directly related to the fl avor and color of foods, but also is helpful for maintaining human health. Since they are easily changed during release from the matrix, digestion, absorption, metabolism and distribution to various tissues in the body, the bioavailability of different dietary polyphenols is different. The most abundant dietary polyphenols do not necessarily represent the highest bioavailability. The absorption and biotransformation of plant polyphenols as well as their bioavailability are one of the current hot research topics. In this article, the gastrointestinal digestion, absorption and metabolism of dietary polyphenols are reviewed based on a mechanism involving intestinal absorption, with the aim to provide valuable information for the research and development of polyphenolics-rich health foods.
Key words:dietary polyphenols; bioavailability; absorption; metabolism; transportation
DOI:10.7506/spkx1002-6630-201707042
中图分类号:Q964.8;R151.2
文献标志码:A
文章编号:1002-6630(2017)07-0266-06
引文格式:
左丹, 廖霞, 李瑶, 等. 基于肠道吸收机制的膳食多酚代谢研究进展[J]. 食品科学, 2017, 38(7): 266-271. DOI:10.7506/ spkx1002-6630-201707042. http://www.spkx.net.cn
ZUO Dan, LIAO Xia, LI Yao, et al. Progress in research on dietary polyphenols metabolism based on a mechanism involving intestinal absorption[J]. Food Science, 2017, 38(7): 266-271. (in Chinese with English abstract)
DOI:10.7506/ spkx1002-6630-201707042. http://www.spkx.net.cn
收稿日期:2016-05-17
基金项目:国家自然科学基金面上项目(31471576);中央高校基本科研业务费专项(XDJK2015D035)
作者简介:左丹(1992—),女,硕士研究生,研究方向为食品化学与营养学。E-mail:804403185@qq.com
*通信作者:明建(1972—),男,教授,博士,研究方向为食品化学与营养学。E-mail:mingjian1972@163.com