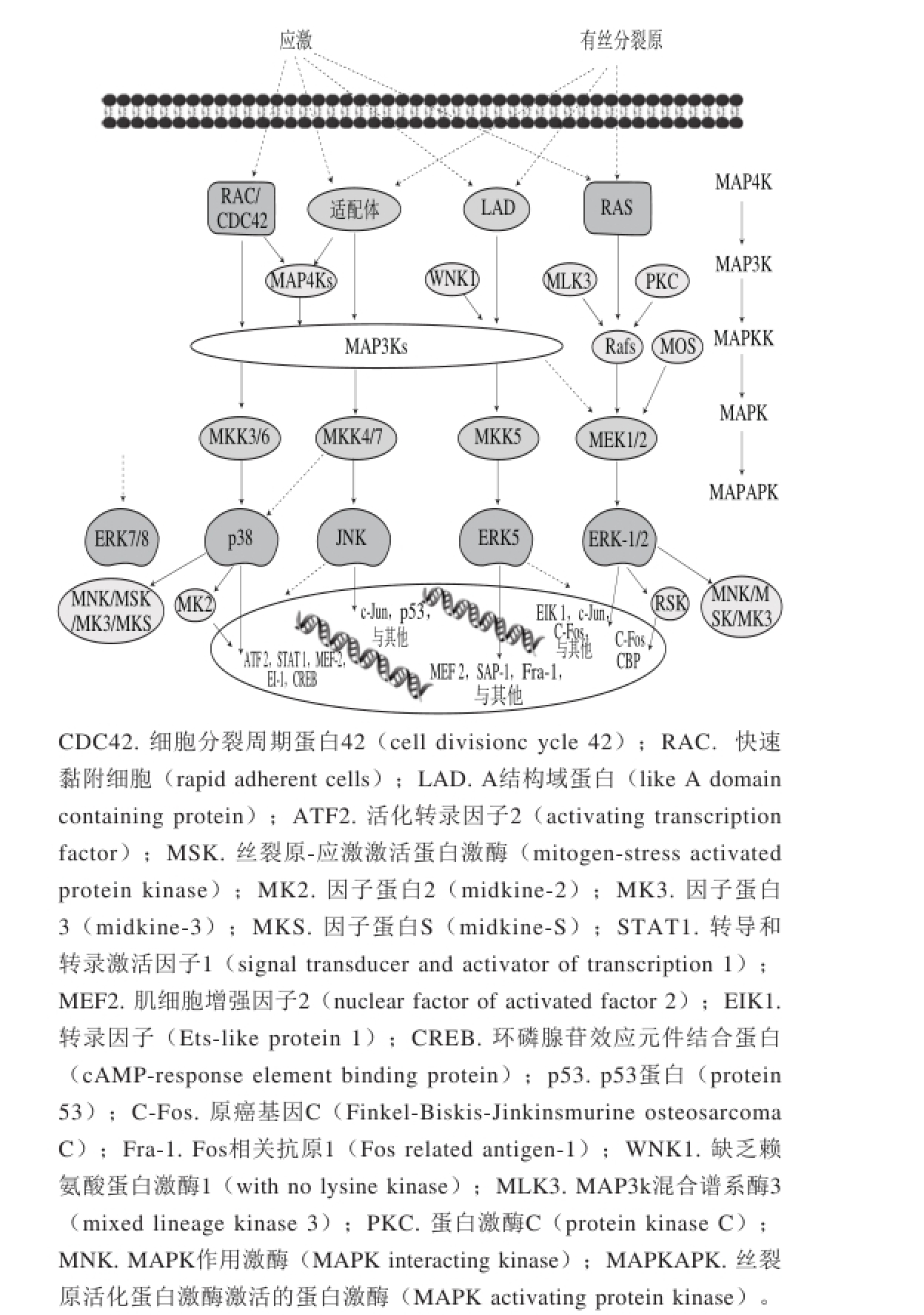
图1 MAPK信号级联示意图[5]
Fig. 1 Schematic diagram of MAPK signaling pathways[5]
李 瑶1,廖 霞1,郑少杰1,王丽颖1,石 芳1,左 丹1,吴素蕊2,明 建1,3,*
(1.西南大学食品科学学院,重庆 400715;2. 中华全国供销合作总社昆明食用菌研究所,云南 昆明 650223;3.重庆市特色食品工程技术研究中心,重庆 400715)
摘 要:肿瘤细胞的生物学过程(增殖、分化、侵袭、迁移)是癌症发生的标志,丝裂原活化蛋白激酶(mitogenactivated protein kinase,MAPK)被证实是调节肿瘤细胞生物学过程的至关重要的信号通路。大量研究表明,天然植物多酚,如茶多酚、白藜芦醇、花青素等对肿瘤细胞有显著的调节作用,植物多酚介导的MAPK通路对肿瘤细胞的调节已经引起国内外学者的广泛兴趣。文章综述了植物多酚通过MAPK信号通路对肿瘤细胞的调控作用,分析了不同多酚对MAPK信号通路的4 条途径(p38、ERK-1/2、ERK5、JNK)的响应机制,旨在为明确植物多酚的抗肿瘤活性及分子机制、开发抗肿瘤保健食品或药物提供参考。
关键词:植物多酚;丝裂原活化蛋白激酶;信号通路;抗肿瘤
癌症是正常细胞在肿瘤发病机制中产生的恶性致瘤性细胞的过程[1]。该过程具有维持肿瘤细胞的增殖信号、避免生长抑制、抗细胞凋亡、使细胞永生、诱导血管生成、激活侵袭和转移、改变能量代谢机制、防止免疫破坏等特点[2]。肿瘤细胞在增殖、凋亡、侵袭和转移等方面的发生机制各不相同。在肿瘤细胞增殖方面,增殖信号对细胞表面受体的表达进行调控以及对信号通路元件的激活是细胞增殖和存活的关键,确定这些途径的组成至关重要。在肿瘤细胞凋亡方面,肿瘤细胞是由致癌信号和过度增殖的相关DNA损伤所造成,其体内具有相应的机制防止凋亡,如肿瘤抑制基因p53的缺失或失活、上调促生存蛋白(B-cell lymphoma-2,Bcl-2)或下调促凋亡蛋白(Bax和BIM),通过相关信号通路对肿瘤细胞凋亡进行调节[3]。在肿瘤细胞侵袭和转移方面,是肿瘤细胞发展为侵袭身体局部组织并转移到末梢位置的过程,激活该过程需要改变肿瘤细胞形状,减少肿瘤细胞在其他细胞和细胞外基质上的附着并增加其运动。而在这些肿瘤细胞在增殖、凋亡、侵袭和转移等过程中,有丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)信号通路的参与介导[4]。
许多研究证实,植物多酚对肿瘤细胞的增殖、凋亡、侵袭和转移等生物学过程有着广泛的调节作用。本文综述了近些年来国内外关于植物多酚介导MAPK信号通路对肿瘤细胞的调控作用机制,总结了不同结构植物多酚在MAPK信号通路相应途径的特点和规律,旨在为研究植物多酚与抗肿瘤之间的关系提供参考。

图1 MAPK信号级联示意图[5]
Fig. 1 Schematic diagram of MAPK signaling pathways[5]
MAPK信号通路,又称有丝分裂原激活蛋白激酶信号通路[6]。它可以通过多个底物如磷酸化转录因子,与细胞骨架相关的细胞和酶来调节细胞各种生理过程,包括炎症、应激、细胞生长发育、分化与死亡[7]。MAPK信号通路主要包括4 种途径(图1):细胞外信号调节的激酶(extracellular regulated protein kinases-1/2,ERK-1/2)、Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)、p38蛋白和细胞外信号调节的激酶5(extracellular regulated protein kinases 5,ERK5)途径。完整的MAPK通路作用过程需要通过三级联转导方式依次激活。这4 种途径需要被具体的MAP2K激活,而MAP2K可以被多种MAP3K所激活,磷酸化MAP3K激活MAP2K,MAP2K进一步磷酸化并激活MAPK。
1.1 MAPK信号通路的主要途径
1.1.1 ERK-1/2途径
细胞外信号调节激酶1和2(ERK-1/2)是MAPK家族的一分子。ERKs是由多种细胞外因子所激活的,其中包括生长因子、激素和神经递质[8]。该细胞外因子可以通过G蛋白偶联受体,氨酸激酶受体或通过离子通道及其他机制[9],进而引发多种细胞内信号响应,导致ERK级联激活。在ERK信号通路中,ERK1或ERK2(ERK-1/2)是由MEK1/2激活,而MEK1/2又依次由Rafs(如A-Raf、B-Raf或Raf-1)激活。ERK-1/2被激活后,紧接着激活MAPKAPK激酶。蛋白激酶GSK3[10]和LKB1[11]是MAPKAPKs直接底物,如此形成了6 个层次的MAPK级联。
ERK-1/2途径对调控肿瘤细胞生长、分化和迁移有一定的作用。通常情况下,促进肿瘤细胞凋亡或抑制细胞增殖需要下调ERK信号通路,如β-榄香烯通过下调MAPK/ERK和PI3K/Akt/mTOR信号通路诱导肾癌786-0细胞的凋亡[12];赖氨酰氧化酶肽通过下调MAPK/ERK信号通路抑制肝肿瘤细胞增殖并诱导其凋亡[13]。然而,并非只有抑制该通路才能促进肿瘤细胞凋亡,如α-干扰素肿瘤坏死因子相关凋亡诱导配体(TNF related apoptosis inducing ligand,TRAIL)通过下调c-casitas B细胞淋巴瘤和上调MAPK/ERK途径诱导胃肿瘤细胞凋亡[14]。
1.1.2 JNK途径
JNK是由“c-Jun氨基末端激酶”衍生而来。上游的MAP2K和MAP3K被丝氨酸/苏氨酸磷酸化所激活,JNK及其同系物被苏氨酸/酪氨酸磷酸化所激活[15-16]。
JNK调控细胞死亡、存活、增殖和分化过程主要有3 个方面的原因:一是激酶的激活或下游目标的选择有助于消除肿瘤细胞,而肿瘤细胞死亡往往涉及到JNK的活化。二是一些肿瘤细胞增殖和转移与JNK的活性有关[17]。三是神经退行性疾病也与JNK介导的细胞死亡相关[18]。然而,JNK对于细胞的生长作用并非都是一致的。有研究表明,JNK能促进人类前列腺癌细胞增殖,而对乳腺癌细胞的影响却不明显,这可能是由于JNK底物的异质性、酶和调节分子的结合作用影响了JNK的活性[19-20]。
1.1.3 p38途径
p38参与了细胞增殖、细胞分化、细胞死亡、细胞迁移和侵袭等一系列复杂的生理生化过程,同时也是控制炎症反应的一条重要通路[21]。
p38/MAPK对调节肿瘤细胞增殖、分化有非常重要的意义。一方面,p38/MAPK的激活可以诱导原发性肿瘤细胞产生细胞上皮-间质转化(epithelial-mesenchymal transition,EMT)导致肿瘤细胞的外渗,造成迁移肿瘤细胞的入侵和迁移[22]。另一方面,p38/MAPK的抑制与肿瘤细胞的抗失巢凋亡特性相关,使得循环肿瘤细胞得以存活,增加恶性肿瘤患者死亡风险[23]。p38/MAPK的高活性与ERK-1/2通路的低活性相结合,可导致肿瘤细胞休眠。研究发现,p38/MAPK被人乳腺上皮细胞的突变基因激活,会导致H-Ras基因特异性细胞在该细胞上进行侵袭和迁移[24-25]。
1.1.4 ERK5途径
MAPK途径中参与细胞调节的激酶ERK5,同其他的激酶一样,也是由多层级联激活的,其中WNK1充当MAP4K激酶,MAP2K主要为MEK5。ERK5能调控肿瘤细胞的增殖、凋亡、侵袭和转移。它是第1个被证明影响HeLa细胞(宫颈癌)增殖作用的MAPK激酶[26]。同样,在乳腺癌细胞CF7和BT474[27]、乳腺上皮细胞系MCF10A[27]、多发性骨髓瘤细胞MM1S[28]的增殖中也有类似作用。MEK5的表达增加了人胚肾细胞HEK-293与前列腺癌细胞LNCaP的增殖[29]。在晚期口腔鳞状细胞癌中,淋巴结的转移也与高水平的MEK5有关[30]。此外ERK5水平下降也可以减少由肝细胞生长因子(hepatocyte growth factor,HGF)诱导的乳腺癌细胞MDA-MB-231迁移[31]。
许多研究证实MAPK信号通路是调节肿瘤细胞生物学过程至关重要的途径。而植物多酚如茶多酚、白藜芦醇、花青素等介导MAPK信号通路对肿瘤细胞的调控已成为国内外研究热点。但是,不同结构的植物多酚在调控肿瘤细胞时究竟响应哪条途径是否有规律可循值得进一步分析和探讨。
表1 植物多酚通过MAPK信号通路调控肿瘤细胞生物学途径
Table 1 MAPK signaling pathways by which plant polyphenols regulate tumor cells
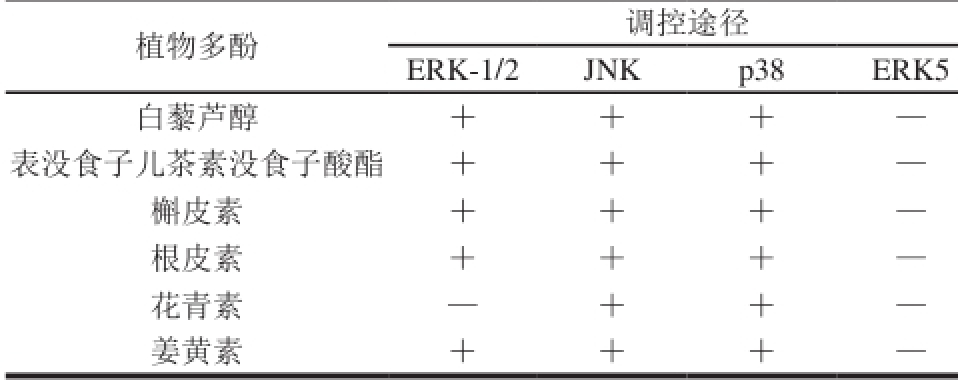
注:+.通过这条途径调控肿瘤细胞;—.不通过这条途径调控肿瘤细胞。
2.1 白藜芦醇
白藜芦醇(resveratrol)是从水果中分离出来的天然多酚类化合物,对多种恶性肿瘤具有抑制效果。早就有研究指出白藜芦醇能快速激活MEK-1、稀疏表达基因(sparse representation-based classif i er,SRC)、基质金属蛋白酶和表皮生长因子受体依赖性模式的MAPK。白藜芦醇通过MAPK信号通路抑制肿瘤细胞增殖的途径主要有两种:一种是通过激活p38途径抑制细胞增殖,一种是抑制ERK途径抑制细胞增殖。
白藜芦醇抗人结肠癌细胞的作用可通过介导上调骨形态发生蛋白(bone morphogenetic protein,BMP9)激活p38/MAPK,从而抑制人结肠癌细胞的增殖并促进其凋亡[32]。且该途径对软骨肉瘤细胞的增殖、迁移和凋亡有同样作用[33]。白藜芦醇对结肠癌细胞增殖的抑制作用也可以通过ERK-1/2通路来调节,经过RES处理的结肠癌细胞,Bax、caspase 3和caspase 9的表达均显著升高,而抗凋亡分子Bcl-2的表达则明显降低;在ERK途径中,白藜芦醇组的RAS、Raf、MEK和ERK-1/2的表达则明显降低,表明白藜芦醇可通过抑制ERK-1/2途径来抑制肿瘤细胞增殖[34]。
2.2 表没食子儿茶素没食子酸酯
绿茶中表没食子儿茶素没食子酸酯(epigallocatechin gallate,EGCG)是茶多酚中含量最丰富、生物学功能最强的活性成分[35]。由于其安全无毒副作用并且可作用于多个组织及器官的特点,EGCG作为肿瘤抑制药物一直备受关注[36]。早期研究发现EGCG对丙二醇甲醚醋酸酯(2-acetoxy-1-methoxypropane,PMA)诱导的ERK和JNK激酶的激活起到一定的抑制作用,通过下调转录因子(activator protein-1,AP-1),从而防止胃肿瘤细胞的侵袭[37]。Yamagata等[38]研究发现结肠肿瘤细胞经过EGCG处理能增加ERK-1/2、JNK1/2、p38α、p38γ和p38β的磷酸化水平。进一步研究发现,使用相应的激酶抑制剂处理,EGCG诱导的细胞凋亡是通过抑制ERK-1/2和Akt或者激活p38/MAPK的活性完成的。还有研究表明EGCG通过介导激活p38/MAPK,下调基质金属蛋白酶(metalloproteinase,MMP2)的表达抑制卵巢癌细胞的增殖和迁移[39]。
2.3 槲皮素
槲皮素(quercetin)是黄酮类化合物,具有抗肿瘤、抗炎和抗氧化等特性[40]。槲皮素可通过产生细胞内活性氧(reactive oxygen species,ROS)和增加sestrin 2表达来激活p38信号通路,进而诱导结肠癌细胞的凋亡[41]。通过诱导体内外细胞的氧化应激,槲皮素也能激活ERK途径,诱导白血病细胞HL-60的凋亡[42]。抑制Ras/MAPK/ERK信号通路则能抑制脑胶质瘤细胞活力和迁移,促进细胞的衰老[43]。特别是对于正常的细胞,如支气管上皮细胞等,槲皮素介导的JNK通路对其生物学过程可表现出相应的反馈调节,槲皮素激活JNK并增加c-Jun和p53依赖性Bax的表达水平诱导支气管上皮细胞的凋亡,同时JNK的失活则会抑制槲皮素减少p53和Bax的表达,进而减轻细胞的凋亡[44]。
2.4 根皮苷
根皮苷(phlorizin)是广泛存在于苹果、梨和各种蔬菜中的植物多酚类物质,具有抗肿瘤活性。根皮苷可以显著上调p53和Bax和多聚ADP核糖聚合酶的裂解,增加caspase-3活性以及活化JNK和p38途径诱导乳腺癌细胞的凋亡[45]。研究发现根皮苷通过p38和JNK1/2途径均能显著地诱导非小细胞性肺癌细胞系A549细胞的凋亡,抑制其增殖[46]。同时,根皮苷还能抑制ERK的磷酸化,抑制12-O-十四烷酰佛波醇-13-醋酸酯(12-O-tetradecanoylphorbol 13-acetate,TPA)诱导的NF-κB活化和环氧化酶(cyclooxygenase,COX-2)表达,而促进肿瘤细胞的凋亡[47]。
2.5 花青素
花青素(anthocyanins)属于黄酮类化合物,具有重要的抗氧化、抗炎及抗癌作用。花青素通过激活p38/MAPK途径,下调抗凋亡蛋白(inhibitor of apoptosis protein,IAPs),促进人结肠癌HCT-116细胞凋亡[48]。花青素的抗肿瘤作用也体现在对JNK途径的激活上,如黑米花色苷抑制JNK的活化,下调基质金属蛋白酶2(matrix metallo proteinase-2,MMP-2)和MMP-9的分泌,从而抑制乳腺癌细胞的转移。有研究表明矢车菊素-3-O-葡萄糖苷(cyanidin-3-O-glucoside chloride,C3G)可以抑制JNK的活性[49],防止肝细胞的凋亡,但对ER K/MA P K和p38/MAPK无抑制作用。花青素除了常见的抗癌活性外,其通过MAPK途径产生的抗炎活性也十分普遍,如红树莓花色苷能够通过抑制MAPK(JNK通路)的活化抑制体外和体内的炎症反应[50]。
2.6 姜黄素
姜黄素(curcumin)是香料姜黄中最重要的功能组分,对于癌症、动脉粥样硬化和糖尿病等疾病具有一定的治疗特性[51]。姜黄素能通过MAPK的ERK-1/2、JNK和p38/MAPK 3 种途径对肿瘤细胞起到调控作用。研究表明小鼠暴露于烟草烟雾(tobacco smoke,TS)12 周后能激活胃和膀胱的ERK-1/2、ERK5、JNK和p38 4 种MAPK途径,并且激活活化蛋白1(activator protein 1,AP-1),而姜黄素能有效地消除TS诱导的胃中的ERK-1/2和JNK/ MAPK途径和膀胱中的ERK-1/2、JNK、p38 MAPK通路的活化,AP-1的活化和EMT的改变,从而对胃癌和膀胱癌起到一定的预防作用[52-53]。虽然姜黄素在体内具有显著的抗肿瘤活性,而药代动力学特征则表明姜黄素的口服生物利用率差,四氢姜黄素(tetra hydro curcuminoids,THC)作为姜黄素的主要代谢产物,能代替p38/MAPK抑制剂有效地扭转MCF-7细胞线粒体膜电位的耗散,并阻止THC介导的Bax表达上调、Bcl-2表达下调、caspase-3的激活及p21表达上调,表明p38/MAPK可能参与THC诱导的MCF-7细胞凋亡[54]。
2.7 其他
除上述多酚之外,还有很多植物多酚同样能通过MAPK途径调控肿瘤细胞的生物过程,而达到防癌抗癌的目的。齐墩果酸和大蒜素可以通过抑制MAPK/ERK信号通路抑制胶质瘤细胞的增殖[55-56];飞燕草色素抑制血管内皮生长因子(vascular endothelial growth factor,VEGF)诱导的ERK-1/2和p38/MAPK磷酸化并降低转录因子CREB和ATF1的表达,从而抑制黑色素瘤细胞的增殖[57]。木犀草素和橄榄叶提取物通过抑制ERK-1/2活性介导的MAPK信号通路对乳腺癌细胞表现出抗增殖活性[58]。间苯三酚通过抑制ERK途径磷酸化,下调下游效应激酶p70S6和翻译起始因子RPS6和eIF4b的表达,从而促进结肠癌癌细胞的凋亡[59]。
本文综述了天然植物多酚通过MAPK信号通路对肿瘤细胞生物学过程的调节作用,植物多酚抗肿瘤作用的机制表现为抑制肿瘤细胞增殖、迁移和侵袭,促进肿瘤细胞的凋亡。MAPK信号通路的几条途径的响应则表现为激活p38、JNK途径和抑制ERK-1/2途径。在这几条途径中,部分多酚可以响应多种激酶同时对一种肿瘤细胞发挥作用,如姜黄素可以同时激活p38、JNK、ERK-1/2途径抑制胃癌和膀胱癌细胞的生长;根皮苷可以同时激活p38和JNK途径诱导非小细胞性肺肿瘤细胞株A549的凋亡。当然,大部分多酚对于一种肿瘤细胞只能激活一条途径,如花青素多通过激活JNK途径调节细胞的生长、侵袭和迁移。
值得注意的是,本文所总结的几类植物多酚在响应MAPK信号通路的不同途径时涉及到ERK5的途径较少,这可能是由于ERK5作为最晚发现的MAPK家族成员,还未得到科学界的广泛重视和应用。但ERK5作为能够调控肿瘤细胞的增殖、迁移和侵袭的MAPK激酶其作用是不能被忽视的。
随着生物学领域对植物多酚通过MAPK信号通路发挥作用的研究进一步深入,MAPK信号通路的4 条途径及其作用机制仍需要进一步阐明,不同结构的植物多酚对这4 种途径的选择也需要进一步深入研究总结,为探究植物多酚发挥其抗肿瘤作用的机制提供参考依据。
参考文献:
[1] HANAHAN D, WEINBERG R A. Hallmarks of cancer: the next generation[J]. Cell, 2011, 144(5): 646-674. DOI:10.1016/ j.cell.2011.02.013.
[2] LOCHHEAD P A, GILLEY R, COOK S J. ERK5 and its role in tumour development[J]. Biochemical Society Transactions, 2012, 40(1): 251-256. DOI:10.1042/BST20110663.
[3] NITHIANANDARAJAH-JONES G N, WILM B, GOLDRING C E P, et al. ERK5: structure, regulation and function[J]. Cellular Signalling, 2012, 24(11): 2187-2196. DOI:10.1016/j.cellsig.2012.07.007.
[4] 陈建勇, 王聪, 王娟, 等. MAPK信号通路研究进展[J]. 中国医药科学, 2011, 1(8): 32-34.
[5] RUBINFELD H, SEGER R. The ERK cascade[J]. Molecular Biotechnology, 2005, 31(2): 151-174. DOI:10.1385/MB:31:2:151.
[6] SEGER R, KREBS E G. The MAPK signaling cascade[J]. The FASEB Journal, 1995, 9(9): 726-735.
[7] BOGOYEVITCH M A, KOBE B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases[J]. Microbiology and Molecular Biology Reviews, 2006, 70(4): 1061-1095. DOI:10.1128/ MMBR.00025-06.
[8] CHUDERLAND D, SEGER R. Protein-protein interactions in the regulation of the extracellular signal-regulated kinase[J]. Molecular Biotechnology, 2005, 29(1): 57-74. DOI:10.1385/MB:29:1:57.
[9] MARMOR M D, SKARIA K B, YARDEN Y. Signal transduction and oncogenesis by ErbB/HER receptors[J]. International Journal of Radiation Oncology Biology Physics, 2004, 58(3): 903-913. DOI:10.1016/j.ijrobp.2003.06.002.
[10] ELDAR-FINKELMAN H, SEGER R, VANDENHEEDE J R, et al. Inactivation of glycogen synthase kinase-3 by epidermal growth factor is mediated by mitogen-activated protein kinase/p90 ribosomal protein S6 kinase signaling pathway in NIH/3T3 cells[J]. Journal of Biological Chemistry, 1995, 270(3): 987-990.
[11] SAPKOTA G P, KIELOCH A, LIZCANO J M, et al. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser 431 by p90 RSK and cAMP-dependent protein kinase, but not its farnesylation at Cys 433, is essential for LKB1 to suppress cell growth[J]. Journal of Biological Chemistry, 2001, 276(22): 19469-19482. DOI:10.1074/jbc.M009953200.
[12] ZHAN Y H, LIU J, QU X J, et al. β-Elemene induces apoptosis in human renal-cell carcinoma 786-0 cells through inhibition of MAPK/ ERK and PI3K/Akt/mTOR signalling pathways[J]. Asian Pacific Journal of Cancer Prevention, 2012, 13(6): 2739-2744. DOI:10.7314/ APJCP.2012.13.6.2739.
[13] ZHENG Y, WANG X, WANG H, et al. Expression of the lysyl oxidase propeptide in hepatocellular carcinoma and its clinical relevance[J]. Oncology Reports, 2014, 31(4): 1669-1676. DOI:10.3892/or.2014.3044.
[14] QU J, ZHAO M, TENG Y, et al. Interferon-α sensitizes human gastric cancer cells to TRAIL-induced apoptosis via activation of the c-CBL-dependent MAPK/ERK pathway[J]. Cancer Biology and Therapy, 2011, 12(6): 494-502. DOI:10.4161/cbt.12.6.15973.
[15] GEE K, LIM W, MA W, et al. Differential regulation of CD44 expression by lipopolysaccharide (LPS) and TNF-α in human monocytic cells: distinct involvement of c-Jun N-terminal kinase in LPS-induced CD44 expression[J]. The Journal of Immunology, 2002, 169(10): 5660-5672. DOI:10.4049/jimmunol.169.10.5660.
[16] BENNETT B L, SASAKI D T, MURRAY B W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase[J]. Proceedings of the National Academy of Sciences, 2001, 98(24): 13681-13686. DOI:10.1073/pnas.251194298.
[17] WOO J H, LIM J H, KIM Y H, et al. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC δ signal transduction[J]. Oncogene, 2004, 23(10): 1845-1853. DOI:10.1038/sj.onc.1207307.
[18] HASHIMOTO Y, TSUJI O, NIIKURA T, et al. Involvement of c-Jun N-terminal kinase in amyloid precursor protein-mediated neuronal cell death[J]. Journal of Neuro Chemistry, 2003, 84(4): 864-877. DOI:10.1046/j.1471-4159.2003.01585.x.
[19] YANG Y M, BOST F, CHARBONO W, et al. C-Jun NH(2)-terminal kinase mediates proliferation and tumor growth of human prostate carcinoma[J]. Clinical Cancer Research, 2003, 9(1): 391-401.
[20] JOHNSON R, SPIEGELMAN B, HANAHAN D, et al. Cellular transformation and malignancy induced by ras require c-Jun[J]. Molecular and Cellular Biology, 1996, 16(8): 4504-4511. DOI:10.1128/MCB.16.8.4504.
[21] 吴向华, 陆云飞. MAPK信号传导通路与乳腺癌关系的研究进展[J].广东医学, 2010, 31(4): 526-528.
[22] TAKEISHI Y, ABE J, LEE J D, et al. Differential regulation of p90 ribosomal S6 kinase and big mitogen-activated protein kinase 1 by ischemia/reperfusion and oxidative stress in perfused guinea pig hearts[J]. Circulation Research, 1999, 85(12): 1164-1172. DOI:10.1161/01.RES.85.12.1164.
[23] SUN W, WEI X, KESAVAN K, et al. MEK kinase 2 and the adaptor protein Lad regulate extracellular signal-regulated kinase 5 activation by epidermal growth factor via Src[J]. Molecular and Cellular Biology, 2003, 23(7): 2298-2308. DOI:10.1128/MCB.23.7.2298-2308.2003.
[24] KIM M S, LEE E J, KIM H R C, et al. p38 Kinase is a key signaling molecule for H-Ras-induced cell motility and invasive phenotype in human breast epithelial cells[J]. Cancer Research, 2003, 63(17): 5454-5461.
[25] KHAJEH M, GHANBARI M. Identif i cation of H-Ras-specif i c motif for the activation of invasive signaling program in human breast epithelial cells[J]. Neoplasia, 2011, 13(2): 98-107. DOI:10.1593/ neo.101088.
[26] KATO Y, TAPPING R I, HUANG S, et al. BMK1/ERK5 is required for cell proliferation induced by epidermal growth factor[J]. Nature, 1998, 395: 713-716. DOI:10.1038/27234.
[27] ESPARÍS-OGANDO A, DÍAZ-RODRÍGUEZ E, MONTERO J C, et al. ERK5 participates in neuregulin signal transduction and is constitutively active in breast cancer cells overexpressing ErbB2[J].Molecular and Cellular Biology, 2002, 22(1): 270-285. DOI:10.1128/ MCB.22.1.270-285.2002.
[28] CARVAJAL-VERGARA X, TABERA S, MONTERO J C, et al. Multifunctional role of ERK5 in multiple myeloma[J]. Blood, 2005, 105(11): 4492-4499.
[29] MEHTA P B, JENKINS B L, MCCARTHY L, et al. MEK5 overexpression is associated with metastatic prostate cancer, and stimulates proliferation, MMP-9 expression and invasion[J]. Oncogene, 2003, 22(9): 1381-1389. DOI:10.1038/sj.onc.1206154.
[30] STICHT C, FREIER K, KNÖPFLE K, et al. Activation of MAP kinase signaling through ERK5 but not ERK1 expression is associated with lymph node metastases in oral squamous cell carcinoma (OSCC) 1, 2, 3[J]. Neoplasia, 2008, 10(5): 462-470. DOI:10.1593/neo.08164.
[31] CASTRO N E, LANGE C A. Breast tumor kinase and extracellular signal-regulated kinase 5 mediate Met receptor signaling to cell migration in breast cancer cells[J]. Breast Cancer Research, 2010, 12(4): R60.
[32] YUAN S X, WANG D X, WU Q X, et al. BMP9/p38 MAPK is essential for the antiproliferative effect of resveratrol on human colon cancer[J]. Oncology Reports, 2016, 35(2): 939-947. DOI:10.3892/ or.2015.4407.
[33] DAI Z, LEI P, XIE J, et al. Antitumor effect of resveratrol on chondrosarcoma cells via phosphoinositide 3-kinase/AKT and p38 mitogen-activated protein kinase pathways[J]. Molecular Medicine Reports, 2015, 12(2): 3151-3155. DOI:10.3892/mmr.2015.3683.
[34] HAO C, JIN Z L, HAI X. MEK/ERK signaling pathway in apoptosis of SW620 cell line and inhibition effect of resveratrol[J]. Asian Pacif i c Journal of Tropical Medicine, 2016, 9(1): 45-48. DOI:10.1016/ j.apjtm.2015.12.010.
[35] WU D, WANG J, PAE M, et al. Green tea EGCG, T cells, and T cellmediated autoimmune diseases[J]. Molecular Aspects of Medicine, 2012, 33(1): 107-118. DOI:10.1016/j.mam.2011.10.001.
[36] 蒋洁琳, 温旭烨, 胡雅琼, 等. 表没食子儿茶素没食子酸酯(EGCG)对癌症细胞信号传导链的影响[J]. 食品科学, 2012, 33(9): 319-325.
[37] KIM H S, KIM M I H, JEONG M I N, et al. EGCG blocks tumor promoter-induced MMP-9 expression via suppression of MAPK and AP-1 activation in human gastric AGS cells[J]. Anticancer Research, 2004, 24(2B): 747-754.
[38] YAMAGATA K, XIE Y, SUZUKI S, et al. Epigallocatechin-3-gallate inhibits VCAM-1 expression and apoptosis induction associated with LC3 expressions in TNFα-stimulated human endothelial cells[J]. Phytomedicine, 2015, 22(4): 431-437. DOI:10.1016/ j.phymed.2015.01.011.
[39] WANG F, CHANG Z, FAN Q, et al. Epigallocatechin 3 gallate inhibits the proliferation and migration of human ovarian carcinoma cells by modulating p38 kinase and matrix metalloproteinase 2[J]. Molecular Medicine Reports, 2014, 9(3): 1085-1089. DOI:10.3892/ mmr.2014.1909.
[40] DAJAS F. Life or death: neuroprotective and anticancer effects of quercetin[J]. Journal of Ethnopharmacology, 2012, 143(2): 383-396. DOI:10.1016/j.jep.2012.07.005.
[41] KIM G T, LEE S H, KIM J I, et al. Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner[J]. International Journal of Molecular Medicine, 2014, 33(4): 863-869. DOI:10.3892/ijmm.2014.1658.
[42] LEE W J, HSIAO M, CHANG J L, et al. Quercetin induces mitochondrial-derived apoptosis via reactive oxygen species-mediated ERK activation in HL-60 leukemia cells and xenog Raft[J]. Archives of Toxicology, 2015, 89(7): 1103-1117. DOI:10.1007/s00204-014-1300-0.
[43] PAN H C, JIANG Q, YU Y, et al. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fi bronectin via the AKT and ERK signalling pathways in human glioma cells[J]. Neurochemistry International, 2015, 80: 60-71. DOI:10.1016/j.neuint.2014.12.001.
[44] LEE K H, YOO C G. Simultaneous inactivation of GSK-3β suppresses quercetin-induced apoptosis by inhibiting the JNK pathway[J]. American Journal of Physiology. Lung Cellular and Molecular Physiology, 2013, 304(11): L782-L789. DOI:10.1152/ ajplung.00348.2012.
[45] KIM M S, KWON J Y, KANG N J, et al. Phloretin induces apoptosis in H-Ras MCF10A human breast tumor cells through the activation of p53 via JNK and p38 mitogen-activated protein kinase signaling[J]. Annals of the New York Academy of Sciences, 2009, 1171(1): 479-483. DOI:10.1111/j.1749-6632.2009.04692.x.
[46] MIN J, LI X, HUANG K, et al. Phloretin induces apoptosis of non-small cell lung carcinoma A549 cells via JNK1/2 and p38 MAPK pathways[J]. Oncology Reports, 2015, 34(6): 2871-2879. DOI:10.3892/or.2015.4325.
[47] SHIN J W, KUNDU J K, SURH Y J. Phloretin inhibits phorbol esterinduced tumor promotion and expression of cyclooxygenase-2 in mouse skin: extracellular signal-regulated kinase and nuclear factor-κB as potential targets[J]. Journal of Medicinal Food, 2012, 15(3): 253-257. DOI:10.1089/jmf.2011.1851.
[48] SHIN D Y, LEE W S, LU J N, et al. Induction of apoptosis in human colon cancer HCT-116 cells by anthocyanins through suppression of Akt and activation of p38-MAPK[J]. International Journal of Oncology, 2009, 35(6): 1499-1504. DOI:10.3892/ijo_00000469.
[49] JIANG X, TANG X, ZHANG P, et al. Cyanidin-3-O-β-glucoside protects primary mouse hepatocytes against high glucose-induced apoptosis by modulating mitochondrial dysfunction and the PI3K/ Akt pathway[J]. Biochemical Pharmacology, 2014, 90(2): 135-144. DOI:10.1016/j.bcp.2014.04.018.
[50] LI L, WANG L, WU Z, et al. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis[J]. Scientif i c Reports, 2014, 4: 6234-6234. DOI:10.1038/srep06234.
[51] AHUJA S, SHANKAR P, BOYLAN M. Curcumin’s therapeutic properties in disease prevention[J]. Agro Food Industry Hi-tech, 2011, 22(5): 26-28.
[52] LIANG Z, WU R, XIE W, et al. Curcumin suppresses MAPK pathways to reverse tobacco smoke-induced gastric epithelialmesenchymal transition in mice[J]. Phytotherapy Research, 2015, 29(10): 1665-1671. DOI:10.1002/ptr.5398.
[53] LIANG Z, WU R, XIE W, et al. Inhibition of tobacco smoke-induced bladder MAPK activation and epithelial-mesenchymal transition in mice by curcumin.[J]. International Journal of Clinical & Experimental Pathology, 2014, 8(5): 4503-4513.
[54] KANG N, WANG M M, WANG Y H, et al. Tetrahydrocurcumin induces G2/M cell cycle arrest and apoptosis involving p38 MAPK activation in human breast cancer cells[J]. Food and Chemical Toxicology, 2014, 67: 193-200. DOI:10.1016/j.fct.2014.02.024.
[55] GUO G, YAO W, ZHANG Q, et al. Oleanolic acid suppresses migration and invasion of malignant glioma cells by inactivating MAPK/ERK signaling pathway[J]. PLoS ONE, 2013, 8(8): e72079-e72079. DOI:10.1371/journal.pone.0072079. eCollection 2013.
[56] CHA J H, CHOI Y J, CHA S H, et al. Allicin inhibits cell growth and induces apoptosis in U87MG human glioblastoma cells through an ERK-dependent pathway[J]. Oncology Reports, 2012, 28(1): 41-48. DOI:10.3892/or.2012.1772.
[57] KERAVIS T, FAVOT L, ABUSNINA A A, et al. Delphinidin inhibits tumor growth by acting on VEGF signalling in end oihelial cells[J]. PLoS ONE, 2015, 10(12): e0145291. DOI:10.1371/journal. pone.0145291.
[58] BARRAJÓN-CATALÁN E, TAAMALLI A, QUIRANTESPINÉ R, et al. Differential metabolomic analysis of the potential antiproliferative mechanism of olive leaf extract on the JIMT-1 breast cancer cell line[J]. Journal of Pharmaceutical and Biomedical Analysis, 2015, 105: 156-162. DOI:10.1016/j.jpba.2014.11.048.
[59] KANG M H, KIM I H, NAM T J. Phloroglucinol induces apoptosis through the regulation of insulin-like growth factor 1 receptor signaling pathways in human colon cancer HT-29 cells[J]. International Journal of Oncology, 2014, 45(3): 1036-1042. DOI:10.3892/ijo.2014.2521.
Plant Polyphenols Exert Antitumor Effect through MAPK Signaling Pathways: A Review
LI Yao1, LIAO Xia1, ZHENG Shaojie1, WANG Liying1, SHI Fang1, ZUO Dan1, WU Surui2, MING Jian1,3,*
(1. College of Food Science, Southwest University, Chongqing 400715, China; 2. Kunming Research Fungi Institute, All China Federation of Supply and Marketing Cooperatives, Kunming 650223, China; 3. Chongqing Engineering Research Center for Special Foods, Chongqing 400715, China)
Abstract:The biological processes of tumor cells such as proliferation, differentiation, invasion and migration are the signs of cancer. The mitogen-activated protein kinase (MAPK) signaling pathway has been proved to be an essential pathway that can adjust the biological processes of tumor cells. A number of studies indicate that natural plant polyphenols, such as tea polyphenols, resveratrol and anthocyanins, have a signif i cant regulatory effect on tumor cells. Plant polyphenols can adjust tumor cells through the MAPK signaling pathway, which has attracted widespread research interest worldwide. This article reviews the regulating effect of plant polyphenols on tumor cells through the MAPK signaling pathway and elucidates the response mechanisms of different polyphenols to the four MAPK signaling pathways (p38, ERK-1/2, ERK5 and JNK pathways), aiming to further clarify the antitumor activity of plant polyphenols and the underlying molecular mechanisms and provide a reference for developing anticancer health foods or drugs.
Key words:plant polyphenols; mitogen-activated protein kinase; signaling pathway; antitumor
DOI:10.7506/spkx1002-6630-201707047
中图分类号:Q964.8;R151.2
文献标志码:A
文章编号:1002-6630(2017)07-0296-06
引文格式:
李瑶, 廖霞, 郑少杰, 等. 植物多酚通过MAPK信号通路调控肿瘤作用机制研究进展[J]. 食品科学, 2017, 38(7): 296-301. DOI:10.7506/spkx1002-6630-201707047. http://www.spkx.net.cn
LI Yao, LIAO Xia, ZHENG Shaojie, et al. Plant polyphenols exert antitumor effect through MAPK signaling pathways: a review[J]. Food Science, 2017, 38(7): 296-301. (in Chinese with English abstract)
DOI:10.7506/spkx1002-6630-201707047. http://www.spkx.net.cn
收稿日期:2016-06-06
基金项目:国家自然科学基金面上项目(31471576);中央高校基本科研业务费专项(XDJK2016E113;XDJK2015D035);“十二五”国家科技支撑计划项目(2013BAD16B01);重庆市特色食品工程技术研究中心能力提升项目(cstc2014pt-gc8001)
作者简介:李瑶(1993—),女,硕士研究生,研究方向为食品化学与营养学。E-mail:420660685@qq.com
*通信作者:明建(1972—),男,教授,博士,研究方向为食品化学与营养学。E-mail:mingjian1972@163.com