表 1 近5 年饮食调节A. muciniphlia丰度的研究
Table 1 A summary of studies on dietary regulation of the intestinal abundance of A. muciniphlia over the past 5 years
注:qPCR.实时荧光定量聚合酶链式反应(quantitive real-time polymerase chain reaction)。
高志鹏,吴 华,耿 欣,宋靖仪,张凯奇,肖俊松*
(北京工商大学 北京食品营养与人类健康高精尖创新中心,北京市食品添加剂工程技术研究中心,北京市植物资源研究开发重点实验室,北京 100048)
摘 要:肠道屏障功能异常与肥胖等一系列慢性代谢性疾病相关。Akkermansia muciniphlia是一种肠道黏液层降解菌,与肠道屏障功能关系密切,其丰度在部分代谢性疾病临床病例和动物模型中变化显著,提示该菌可能参与代谢,但具体作用机制有待明确和归纳。本文从A. muciniphlia与肠道黏液层、代谢疾病以及肠道免疫关系这3 个角度,综述了近年来相关研究进展,试图探讨该菌对肠道屏障功能的影响和作用机制,为通过饮食预防慢性代谢疾病提供新的思路。
关键词:Akkermansia muciniphlia;肠道屏障功能;慢性代谢疾病;丰度
肠道黏液层是肠道的物理屏障之一,可以阻止肠内细菌和抗原物质与肠上皮细胞直接接触。受损的肠屏障会导致某些肠道菌群及其分泌的抗原进入循环系统,引起全身慢性炎症反应,造成肥胖、胰岛素抵抗和2型糖尿病等代谢性疾病[1]。目前有许多证据表明,肠道菌群是影响宿主代谢的关键因素之一[2]。Akkermansia muciniphlia是一种胃肠道黏液层降解菌,定植于肠道黏液层[3],其在肥胖、2型糖尿病、肠炎等疾病患者肠道中丰度变化显著[4-5],表明该菌与宿主代谢类疾病有着密切关系,但目前其与肠道屏障功能的关系并未完全阐明,该菌影响宿主代谢疾病的机制也值得进一步研究。本文总结了肠道黏液层环境特性,分析了A. muciniphlia与黏液层的相互作用,最后通过A. muciniphlia在代谢疾病和机体免疫中的丰度变化及饮食调控来讨论其发挥作用的途径和机制,并对该菌可能成为新一代益生菌的前景进行了展望。
黏液层主要由肠上皮杯状细胞分泌的MUC2黏蛋白组成,呈高度含水(水分体积分数95%)的凝胶状,同时也含有盐、脂质和涉及免疫应答的蛋白质,如生长因子、免疫球蛋白、溶菌酶和其他肠蛋白[6-7],并作为保护层覆盖许多人和动物器官(包括整个胃肠道)中的上皮细胞[8]。上皮细胞的形态改变、连接缝隙增大或开放数目增加都会影响上皮细胞屏障功能,使其通透性增加。异常的肠通透性将无法阻止肠道中各种抗原类物质如内毒素、鞭毛蛋白和食物中的致敏原等进入体内,从而诱发慢性炎症反应,最终导致肥胖、2型糖尿病等一系列疾病[1]。因此,受损的肠屏障功能极有可能是造成代谢疾病的共同诱因。研究发现,黏液层由内黏液层和外黏液层构成:内黏液层由杯状细胞持续分泌生成,与肠上皮细胞紧密连接,没有微生物定植;外黏液层相对松散,适合微生物定植,并持续被微生物水解,保持了黏液层分泌和水解的动态平衡[6]。定植在黏液层的微生物可以参与黏蛋白部分翻译后修饰,改变黏液层厚度,从而影响肠屏障功能[9]。
适应黏液层复杂的糖蛋白环境[11]。这些酶也可能参与A. m u c i n i p h l i a与黏液层的黏附过程。在两种A. muciniphlia分泌的黏蛋白酶(硫酸酯酶由Amuc_0953编码、糖苷酶由Amuc_2164编码)中发现了一种称为BACON(bacteroidetes-associated carbohydrate-binding often N-terminal)的结构域,该结构可能参与黏蛋白酶与黏蛋白的结合过程[11]。A. muciniphlia可以在利用黏液层蛋白维持自身代谢的同时,不影响黏液层正常生理功能。研究发现,无菌小鼠肠道黏液层也存在松散的外层结构,说明体内存在内源性黏液层降解酶,但同时发现,无菌小鼠盲肠黏液层增厚,引起囊肿[6],表明内源性蛋白酶不能维持正常的黏液层厚度。而在无菌小鼠肠道中定植A. muciniphlia后,便可以恢复黏液层过厚引起的盲肠囊肿,说明其可以和内源性蛋白酶一起维持黏液层正常生理厚度。A. muciniphlia可以利用黏液层,但不会导致黏液层厚度减少[2],可能是其在代谢的过程中刺激上皮杯状细胞分泌更多黏蛋白,从而保持黏液层厚度处于一种动态平衡。
A. muciniphlia的表面膜蛋白在细菌定植过程中与黏液层接触密切,有助于该菌感知外部环境,调节自身代谢。Ottman等[12]使用蛋白质组学和计算分析的综合方法鉴定和表达A. muciniphlia蛋白质,确定了79 个潜在的外膜蛋白和相关蛋白,继而分别在有黏蛋白和无黏蛋白添加的培养基上培养,发现A. muciniphlia中有23 个蛋白基因由于A. muciniphlia培养条件的不同产生丰度差异,这些差异蛋白在A. muciniphlia与黏液层作用时发挥的具体作用还需要进一步研究。
黏液层为定植的微生物提供初始黏附位点、营养源和基质,其状态会影响A. muciniphlia的定植。当黏液层变薄或缺损时,黏附其上的A. muciniphlia丰度会显著下降。例如发生溃疡性结肠炎时,A. muciniphlia的相对丰度下降为0.02%左右[13]。A. muciniphlia与黏液层的相互作用可以维持正常的肠屏障功能,但两者的因果关系还不明晰,值得进一步研究证实。
A. muciniphlia是一种胃肠道黏液层降解菌,为革兰氏阴性的严格厌氧菌,属疣微菌门[10]。目前,已知疣微菌门下至少有8 种不同的Akkermansia定植于人体肠道,其中A. muciniphlia是优势菌群,占疣微菌门的83%,丰度在0.01%~4.00%[11]。因其是疣微菌门下唯一可以在体外培养的菌种,逐渐成为了国内外研究的热点[3]。
A. muciniphlia可以降解并利用黏蛋白,维持自身代谢。通过生物信息学分析预测,该菌可以产生61 种与黏蛋白降解相关的酶,如糖苷酶、硫酸酯酶等,称为黏蛋白酶,均是可以作用于黏蛋白的胞外酶,以
肠道菌群参与调节众多代谢途径,影响肥胖症及其他慢性代谢性疾病的发展[14]。近年的研究表明,在肥胖和2型糖尿病患者的肠道菌群中A. muciniphlia丰度变化显著,表明该菌可能参与疾病的进程。
孕期肥胖妇女肠道菌群中A. muciniphlia丰度下降,每克粪便中A. muciniphlia基因组相对含量由8.54降至8.12[15]。同时有研究指出,肥胖女性粪便A. muciniphlia相对丰度与胰岛素抵抗标志物呈负相关[16]。肥胖个体肠道中A. muciniphlia的定植较少[17],且与身体质量指数呈负相关[18]。经过16 周减肥饮食后,A. muciniphlia丰度随体质量减小而上升[19]。在动物模型中,饮食诱导的肥胖可以导致A. muciniphlia丰度下降83 倍[20]。这些研究表明,减轻体质量有利于A. muciniphlia的定植。
在2型糖尿病个体中可以观察到肠道菌群生态失调,A. muciniphlia的丰度变化尤为显著。A. muciniphlia在2型糖尿病个体中具有更低的丰度[4]。在2型糖尿病发病之前,患者体内与发病过程相关的肠道氧化应激状态以及肠道菌群的组成和功能发生变化(A. muciniphlia的丰度降低),这些现象有助于2型糖尿病的早期诊断,因此A. muciniphlia可能是2型糖尿病潜在的生物标记[21]。在动物模型中,由瘦素缺陷小鼠(ob/ob)诱导的糖尿病模型中,A. muciniphlia丰度下降为对照组的1/3 300[2]。
上述研究证实,A. muciniphlia在参与肥胖和2型糖尿病能量代谢调节过程中起着重要作用。A. muciniphlia可以改变小鼠体内内源性大麻素含量,而内源性大麻素系统参与葡萄糖和能量代谢的控制[22]。胃旁路手术后,胰高血糖素样肽1分泌增加,同时A. muciniphlia的丰度上升,2型糖尿病症状也得到改善[23]。二甲双胍广泛应用于治疗2型糖尿病,其作用机制与肠道菌群有直接关系。二甲双胍可以恢复肠道中的紧密连接蛋白闭合蛋白-1水平,逆转升高的肠道通透性和血清脂多糖(lipopolysaccharides,LPS)水平,增强胰岛素信号转导,并增加A. muciniphlia的丰度,在高脂膳食的小鼠中发挥降血糖作用[24]。在摄入二甲双胍的糖尿病人中同样检测到A. muciniphlia丰度增加[25]。A. muciniphlia缺乏会导致肠屏障功能异常,使其他微生物代谢生成的内毒素进入血液并引起与肥胖有关的慢性炎症[17],血清LPS水平与A. muciniphlia在盲肠中的丰度呈逆相关。A. muciniphlia可能是通过影响肠屏障功能进而影响能量代谢,但具体的作用机制还不明晰,有待深入研究。
肠道黏液层是一道肠道细菌和内毒素不能自由逾越的物理屏障,也是肠道免疫的前沿。在相对完整的黏液层环境中,A. muciniphlia的定植可以发挥积极作用。通过对载脂蛋白E缺陷型小鼠的研究发现,A. muciniphlia可以恢复黏液层,减少循环系统中LPS水平[26]。对高脂膳食小鼠灌胃A. muciniphlia,可增加内源性大麻素水平,降低体内炎症反应[2]。而A. muciniphlia在无菌小鼠体内定植后,增强了与免疫反应相关的黏液层基因的表达[27]。
A. muciniphlia可以刺激宿主产生免疫应答。Greer等[28]研究了干扰素γ(interferons γ,IFNγ)、A. muciniphlia和宿主葡萄糖耐受的关系,发现A. muciniphlia丰度与IFNγ水平呈负相关,进而发现在IFNγ基因敲除小鼠中,其仅当A. muciniphlia存在时表现出葡萄糖耐受,最后找到了调控A. muciniphlia丰度的关键基因——免疫相关GTP酶M1,说明A. muciniphlia在宿主葡萄糖耐受调控中起到重要作用。A. muciniphlia还可以刺激肠黏膜潘氏细胞分泌抗菌肽(主要是胰岛再生源蛋白3γ),维持肠道屏障功能[29]。Everard等[2]用A. muciniphlia处理高脂膳食小鼠后发现其结肠内抗菌肽胰岛再生源蛋白3γ含量增多,但是用热灭活的A. muciniphlia处理却没有类似的现象发生。A. muciniphlia表面膜蛋白也可以引起机体免疫调控。一种在该菌膜表面大量表达的鞭毛样蛋白Amuc_1100被直接证实涉及宿主免疫调控,该蛋白可以刺激Toll样受体(Toll like receptors,TLR)2和TLR4产生特殊的细胞因子,造成白介素-10含量增加[30],而且在经过巴氏杀菌后,其结构功能依旧稳定[31]。
有证据表明A. muciniphlia在肠道内定植的相对丰度往往和局部炎症程度呈负相关。溃疡性结肠炎病人体内A. muciniphlia定植减少,但与黏液层相关的致病菌数量增多[5]。Casellas等[32]指出,肠道菌群结构变化和A. muciniphlia数量减少与溃疡性结肠炎的复发过程有关。急性阑尾炎黏膜病变严重程度也和A. muciniphlia丰度呈负相关[33]。
值得注意的是,A. muciniphlia影响肠炎疾病是与其他菌群的相互作用的共同结果。在鼠伤寒沙门氏菌诱发的肠道炎症中,A. muciniphlia干扰黏膜重建,加剧了炎症的发生[34],其原因可能是A. muciniphlia分解的黏蛋白产物增多,为其他肠道致病菌提供了繁殖所需的营养物质[5]。
这些研究表明,A. muciniphlia与机体免疫过程有密切的联系,往往可以改善体内炎症反应。其不仅可以直接刺激机体产生免疫应答,还可以通过作用于黏液层,改善肠道屏障功能,减少肠腔中抗原类物质进入循环系统,从而减轻炎症。在炎症性肠炎病例中,往往伴随着不同程度的肠黏膜损伤,局部黏液层结构破坏可能是A. muciniphlia丰度显著下降的原因之一。是否能够通过恢复黏液层正常生理状态增加A. muciniphlia定植丰度,从而改善机体免疫,起到治疗肠炎疾病的效果,值得进一步尝试。
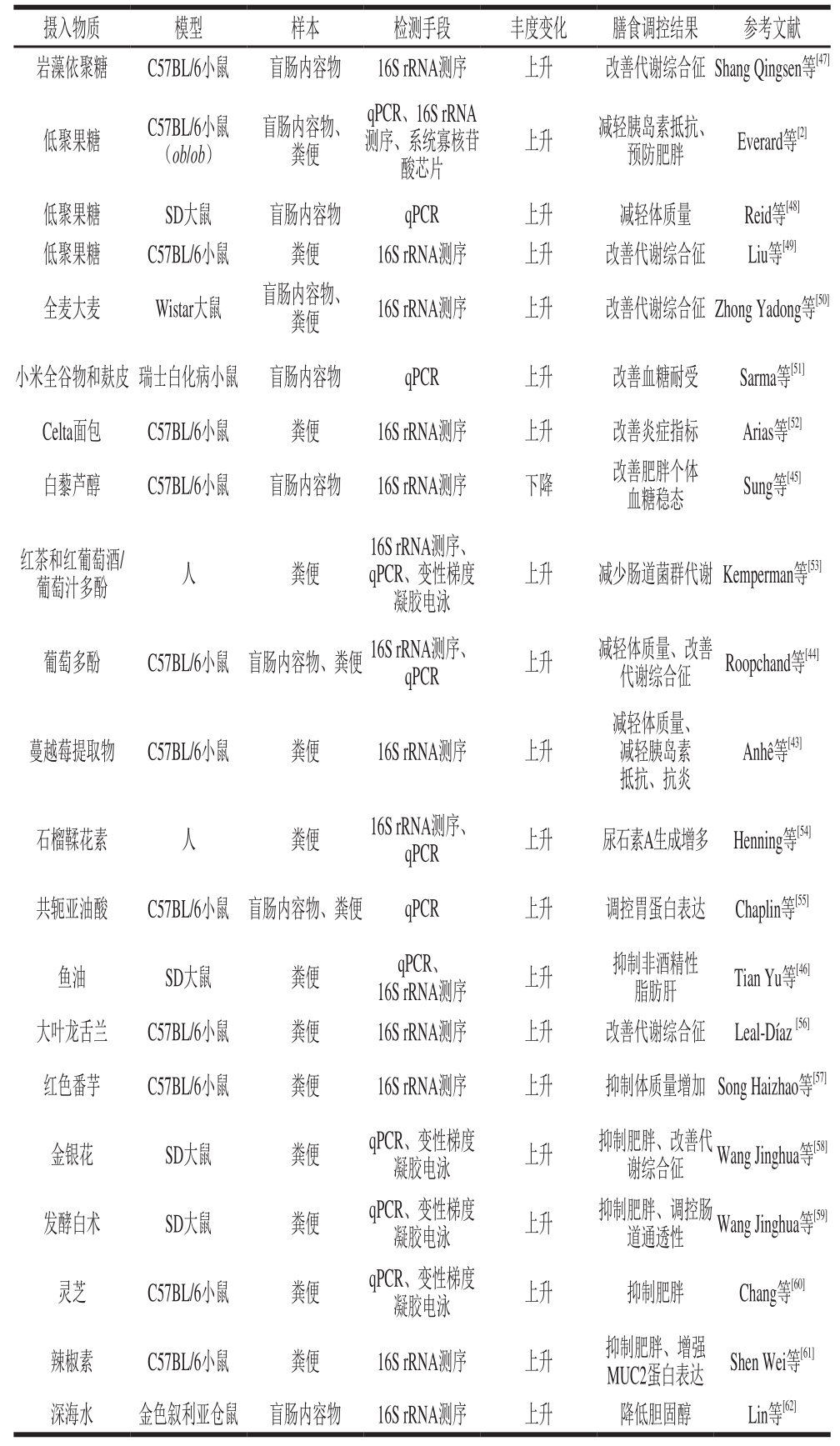
A. muciniphlia可能是饮食调节代谢反应的关键微生物。表1总结了近年来饮食调节A. muciniphlia丰度的研究。
表 1 近5 年饮食调节A. muciniphlia丰度的研究
Table 1 A summary of studies on dietary regulation of the intestinal abundance of A. muciniphlia over the past 5 years

注:qPCR.实时荧光定量聚合酶链式反应(quantitive real-time polymerase chain reaction)。
由表1可知,膳食纤维、低聚寡糖、植物多酚、不饱和脂肪酸、部分中药材等均可以对A. muciniphlia的丰度产生显著影响,同时改善了机体代谢功能,说明通过特定饮食或药理学干预肠道微生物群组成和丰度可有利地影响宿主代谢[35]。
益生元是调节A. muciniphlia丰度的关键因素。A. muciniphlia可以分解膳食纤维,产生与宿主代谢相关的信号分子。长链阿拉伯木聚糖和菊粉可以使盲肠中的Akkermansia丰度下降,使粪便中A. muciniphlia的丰度增加。同时,A. muciniphlia等肠道菌群可以分解阿拉伯木聚糖和菊粉以及黏蛋白降解物,产生短链脂肪酸等有益的代谢物,使整个肠道健康得到改善[36]。
乙酸盐可以帮助改善肠屏障功能[37],丁酸盐则具有抗炎的作用[38],这些物质被发现同时具有抑制I型糖尿病的功能[39]。值得注意的是,短链脂肪酸及其代谢产物的含量可能是调控A. muciniphlia丰度的因素之一。早期研究在禁食状态的叙利亚仓鼠粪便中发现A. muciniphlia数量上升,伴随着肠内短链脂肪酸含量和菌群总量下降,而在短链脂肪酸和菌群总量都没有下降的冬眠个体中,A. muciniphlia数量没有显著变化[40]。
膳食调控A. muciniphlia的机制可能与肠道屏障功能有关。饮食中若缺少膳食纤维,某些肠道微生物会分解黏液层上的多糖,破坏黏液层,以此维持自身代谢[41]。高脂膳食会使黏液层变薄,破坏肠道屏障功能,减少A. muciniphlia在肠道的定植。Su Danmei等[42]发现在高脂膳食及VD缺乏的双重作用下,潘氏细胞α防御素(α-defensin5,DEFA5)水平下降,肠上皮细胞MUC2和紧密连接蛋白的表达量减少,肠道通透性增加,同时A. muciniphlia丰度下降,而在直接摄入DEFA5后,A. muciniphlia丰度上升,并且代谢紊乱得到改善。由此可见,不合理的膳食可以影响黏液层的生理状态,从而降低A. muciniphlia的丰度。
A. muciniphlia的丰度可能与肠道上皮氧化还原状态有关。植物多酚和不饱和脂肪酸均有很强的抗氧化性,可以保护肠道黏液层,增加A. muciniphlia的定植。蔓越莓提取物[43]、葡萄多酚[44]、白藜芦醇[45]都可以增加A. muciniphlia的定植。紫苏油含有不饱和脂肪酸,具有抗氧化的效果,对高脂肪饮食诱导的非酒精性脂肪肝病和肠道生态失调产生保护作用,与高脂膳食组相比,在饲喂鱼油的动物中,A. muciniphlia的相对丰度显著增加[46]。
由此可见,膳食是调控A. muciniphlia的关键因素,其中的机理可能是多方面的。益生元类物质能够直接成为A. muciniphlia的作用底物,产生的短链脂肪酸等有益代谢产物可以改善肠道屏障功能。另外,具有抗氧化能力的物质可以缓解肠道氧化应激,同样起到保护肠道屏障功能的作用,从而增加了A. muciniphlia的丰度。是否可以通过合理膳食来修复肠道屏障功能最终预防慢性代谢疾病是值得研究的方向。
肠道黏液层是构成肠道物理屏障的重要部分,其与定植的微生物互作影响肠道通透性,最终影响慢性代谢疾病的发展。黏液层一方面作为物理屏障,防止肠道内容物与肠道上皮细胞直接接触,避免微生物引起炎症反应;另一方面也同时为肠道菌群提供栖息和繁殖的场所,松散的黏液外层提供肠道菌群必要的能源物质和适宜的生存环境。因此,肠道黏液层、肠道菌群和慢性代谢疾病三者的关系正受到越来越多的关注。A. muciniphlia的丰度和宿主健康状态密切相关,肠道屏障功能是连接两者的通路之一,但其与肠道屏障功能的关系仍未完全阐明,明确A. muciniphlia在改善肠道屏障功能中的贡献是值得研究的方向。
A. muciniphlia具有成为新一代益生菌的潜质,有很好的应用前景。越来越多的研究表明,A. muciniphlia可以影响能量代谢,改善慢性代谢疾病,虽然其中的机制还不清楚,但是已经证实其具有改善高脂饮食引起的代谢紊乱的能力。但是目前大部分实验均属于动物实验,其在人群中的效果有待证实,对于不同人群可能产生的效果差异也值得进一步研究。关于该菌的毒理学实验还鲜见报道,在食品中的最适添加量以及长期摄入对肠道稳态的影响也亟待确定。目前还鲜有A. muciniphlia致病的报道,虽然该菌属于黏液层降解菌,但其仅定植于外黏液层,不会对肠道屏障功能产生负面影响。
总体而言,A. muciniphlia与肠道屏障功能有着密切的关系,其在不同生理状态的个体中的丰度变化,可以反映机体的肠道健康。其与机体代谢、肠免疫等有紧密的联系,通过饮食可以调节A. muciniphlia的丰度,改善宿主代谢疾病,而其中的机理以及黏液层在此发挥的作用值得进一步研究。
参考文献:
[1] CANI P D, BIBILONI R, KNAUF C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inf l ammation in high-fat dietinduced obesity and diabetes in mice[J]. Diabetes, 2008, 57(6): 1470-1481. DOI:10.2337/db07-1403.
[2] EVERARD A, BELZER C, GEURTS L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls dietinduced obesity[J]. Proceedings of the National Academy of Sciences,2013, 110(22): 9066-9071. DOI:10.1073/pnas.1219451110.
[3] DERRIEN M, BELZER C, DE VOS W M. Akkermansia muciniphila and its role in regulating host functions[J]. Microbial Pathogenesis,2016, 106: 171-181. DOI:10.1016/j.micpath.2016.02.005.
[4] YASSOUR M, LIM M Y, YUN H S, et al. Sub-clinical detection of gut microbial biomarkers of obesity and type 2 diabetes[J]. Genome Medicine, 2016, 8(1): 17. DOI:10.1186/s13073-016-0271-6.
[5] PNG C W, LINDÉN S K, GILSHENAN K S, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria[J]. American Journal of Gastroenterology,2010, 105(11): 2420-2428. DOI:10.1038/ajg.2010.281.
[6] JOHANSSON M E V, PHILLIPSON M, PETERSSON J, et al.The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria[J]. Proceedings of the National Academy of Sciences, 2008, 105(39): 15064-15069. DOI:10.1073/pnas.0803124105.
[7] PEARSON J P, ALLEN A, PARRY S. A 70 000-molecular-weight protein isolated from purified pig gastric mucus glycoprotein by reduction of disulphide bridges and its implication in the polymeric structure[J]. The Biochemical Journal, 1981, 197(1): 155-162.DOI:10.1042/bj1970155.
[8] DERRIEN M, VAN PASSEL M W, VAN DE BOVENKAMP J H, et al. Mucin-bacterial interactions in the human oral cavity and digestive tract[J]. Gut Microbes, 2011, 1(4): 254-268. DOI:10.4161/gmic.1.4.12778.
[9] 冯泽猛, 包显颖, 印遇龙. 胃肠道黏液层中Akkermansia muciniphila的定殖及其与宿主的相互作用[J]. 中国农业科学, 2016, 49(8):1577-1584.
[10] DERRIEN M, VAUGHAN E E, PLUGGE C M, et al. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium[J]. International Journal of Systematic & Evolutionary Microbiology, 2004, 54(Pt 5): 1469-1476. DOI:10.1099/ijs.0.02873-0.
[11] VAN PASSEL M W J, KANT R, ZOETENDAL E G, et al. The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes[J]. PLoS ONE, 2011, 6(3): e16876. DOI:10.1371/journal.pone.0016876.
[12] OTTMAN N, HUUSKONEN L, REUNANEN J, et al. Characterization of outer membrane proteome of Akkermansia muciniphila reveals sets of novel proteins exposed to the human intestine[J]. Frontiers in Microbiology, 2016, 7: 1157. DOI:10.3389/fmicb.2016.01157.
[13] ZHANG Z, WU X Y, CAO S Y, et al. Caffeic acid ameliorates colitis in association with increased Akkermansia population in the gut microbiota of mice[J]. Oncotarget, 2016, 7(22): 31790-31799.DOI:10.18632/oncotarget.9306.
[14] ARON-WISNEWSKY J, CLEMENT K. The effects of gastrointestinal surgery on gut microbiota: potential contribution to improved insulin sensitivity[J]. Current Atherosclerosis Reports, 2014, 16(11): 454.DOI:10.1007/s11883-014-0454-9.
[15] SANTACRUZ A, COLLADO M C, GARCÍA-VALDÉS L, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women[J]. British Journal of Nutrition, 2010, 104(1): 83-92. DOI:10.1017/s0007114510000176.
[16] BRAHE L K, LE C E, PRIFTI E, et al. Specif i c gut microbiota features and metabolic markers in postmenopausal women with obesity[J].Nutrition & Diabetes, 2015, 5(6): 159. DOI:10.1038/nutd.2015.9.
[17] BRADLOW H L. Obesity and the gut microbiome: pathophysiological aspects[J]. Hormone Molecular Biology & Clinical Investigation,2014, 17(1): 53-61. DOI:10.1515/hmbci-2013-0063.
[18] WICKER T, KELLER B. The gut microbiota of Colombians differs from that of Americans, Europeans and Asians[J]. BMC Microbiology,2014, 14(1): 1-14. DOI:10.1186/s12866-014-0311-6.
[19] REMELY M, TESAR I, HIPPE B, et al. Gut microbiota composition correlates with changes in body fat content due to weight loss[J]. Beneficial Microbes, 2015, 6(4): 431-439. DOI:10.3920/BM2014.0104.
[20] EVERARD A, LAZAREVIC V, DERRIEN M, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice[J]. Diabetes, 2011,60(11): 2775-2786. DOI:10.2337/db11-0227.
[21] ZHANG X Y, SHEN D Q, FANG Z W, et al. Human gut microbiota changes reveal the progression of glucose intolerance[J]. PLoS ONE,2013, 8(8): e71108. DOI:10.1371/journal.pone.0071108.
[22] CANI P D, GEURTS L, MATAMOROS S, et al. Glucose metabolism:focus on gut microbiota, the endocannabinoid system and beyond[J].Diabetes and Metabolism, 2014, 40(4): 246-257. DOI:10.1016/j.diabet.2014.02.004.
[23] YAN M, SONG M M, BAI R X, et al. Effect of Roux-en-Y gastric bypass surgery on intestinal Akkermansia muciniphila[J]. World Journal of Gastrointestinal Surgery, 2016, 8(4): 301-307. DOI:10.4240/wjgs.v8.i4.301.
[24] ZHOU Z Y, REN L W, ZHAN P, et al. Metformin exerts glucoselowering action in high-fat fed mice via attenuating endotoxemia and enhancing insulin signaling[J]. Acta Pharmacologica Sinica, 2016,37(8): 1063-1075. DOI:10.1038/aps.2016.21.
[25] DE LA CUESTA-ZULUAGA J, MUELLER N T, CORRALESAGUDELO V, et al. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut[J]. Diabetes Care, 2017, 40(1): 54-62. DOI:10.2337/dc16-1324.
[26] LI J, LIN S Q, VANHOUTTE P M, et al. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemiainduced inflammation in Apoe−/− mice [J]. Circulation, 2016,133(24): 2434-2446. DOI:10.1161/CIRCULATIONAHA.115.019645.
[27] DERRIEN M, VAN BAARLEN P, HOOIVELD G, et al. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader Akkermansia muciniphila[J]. Frontiers in Microbiology, 2011, 2: 166. DOI:10.3389/fmicb.2011.00166.
[28] GREER R L, DONG X X, MORAES A C F, et al. Akkermansia muciniphila mediates negative effects of IFNγ on glucose metabolism[J]. Nature Communications, 2016, 7: 13329.DOI:10.1038/ncomms13329.
[29] BEVINS C L, SALZMAN N H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis[J]. Nature Reviews Microbiology, 2011, 9(5): 356-368. DOI:10.1038/nrmicro2546.
[30] OTTMAN N, REUNANEN J, MEIJERINK M, et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function[J]. PLoS ONE, 2017, 12(3): e0173004. DOI:10.1371/journal.pone.0173004.
[31] PLOVIER H, EVERARD A, DRUART C, et al. A purif i ed membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice[J]. Nature Medicine,2016, 23(1): 107. DOI:10.1038/nm.4236.
[32] CASELLAS F, BORRUEL N, MANICHANH C, et al. OP022 low microbial gene diversity and depletion of Akkermansia muciniphila is associated with a relapsing course of ulcerative colitis[J]. Journal of Crohn's and Colitis, 2014, 8(Suppl 1): S12-S13. DOI:10.1016/S1873-9946(14)60023-4.
[33] SWIDSINSKI A, DORFFEL Y, LOENING-BAUCKE V, et al. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum[J]. Gut, 2011, 60(1): 34-40. DOI:10.1136/gut.2009.191320.
[34] GANESH B P, KLOPFLEISCH R, LOH G, et al. Commensal Akkermansia muciniphila exacerbates gut inf l ammation in salmonella typhimurium-infected gnotobiotic mice[J]. PLoS ONE, 2013, 8(9):e74963. DOI:10.1371/journal.pone.0074963.
[35] 宋雪琳, 李雅梅, 肖俊松, 等. 葡萄籽原花青素对营养肥胖模型大鼠肠道菌群的影响[J]. 食品科学技术学报, 2015, 33(5): 39-46.DOI:10.3969/j.issn.2095-6002.2015.05.007.
[36] VAN DEN ABBEELE P, GÉRARD P, RABOT S, et al.Arabinoxylans and inulin differentially modulate the mucosal and luminal gut microbiota and mucin-degradation in humanized rats[J].Environmental Microbiology, 2011, 13(10): 2667-2680. DOI:10.1111/j.1462-2920.2011.02533.x.
[37] FUKUDA S, TOH H, HASE K, et al. Bif i dobacteria can protect from enteropathogenic infection through production of acetate[J]. Nature,2011, 469: 543-547. DOI:10.1038/nature09646.
[38] THORBURN A, MACIA L, MACKAY C. Diet, metabolites, and“Western-Lifestyle” inf l ammatory diseases[J]. Immunity, 2014, 40(6):833-842. DOI:10.1016/j.immuni.2014.05.014.
[39] MARIÑO E, RICHARDS J L, MCLEOD K H, et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes[J]. Nature Immunology, 2017, 18(5): 552-562.DOI:10.1038/ni.3713.
[40] SONOYAMA K, FUJIWARA R, TAKEMURA N, et al. Response of gut microbiota to fasting and hibernation in Syrian hamsters[J].Applied and Environmental Microbiology, 2009, 75(20): 6451-6456.DOI:10.1128/aem.00692-09.
[41] DESAI M S, SEEKATZ A M, KOROPATKIN N M, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility[J]. Cell, 2016, 167(5): 1339-1353.DOI:10.1016/j.cell.2016.10.043.
[42] SU Danmei, NIE Yuanyang, ZHU Airui, et al. Vitamin D signaling through induction of paneth cell defensins maintains gut microbiota and improves metabolic disorders and hepatic steatosis in animal models[J]. Frontiers in Physiology, 2016, 7: 498. DOI:10.3389/fphys.2016.00498.
[43] ANHÊ F F, ROY D, PILON G, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inf l ammation in association with increased Akkermansia spp.population in the gut microbiota of mice[J]. Gut, 2014, 64(6): 872-883.DOI:10.1136/gutjnl-2014-307142.
[44] ROOPCHAND D E, CARMODY R N, KUHN P, et al. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome[J].Diabetes, 2015, 64(8): 2847-2858. DOI:10.2337/db14-1916.
[45] SUNG M M, KIM T T, DENOU E, et al. Improved glucose homeostasis in obese mice treated with resveratrol is associated with alterations in the gut microbiome[J]. Diabetes, 2016, 66(2): 418-425.DOI:10.2337/db16-0680.
[46] TIAN Yu, WANG Hualin, YUAN Fahu, et al. Perilla oil has similar protective effects of fish oil on high-fat diet-induced nonalcoholic fatty liver disease and gut dysbiosis[J]. BioMed Research International,2016, 2016: 1-11. DOI:10.1155/2016/9462571.
[47] SHANG Qingsen, SONG Guanrui, ZHANG Meifang, et al.Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice[J]. Journal of Functional Foods, 2017, 28: 138-146.DOI:10.1016/j.jff.2016.11.002.
[48] REID D T, ELLER L K, NETTLETON J E, et al. Postnatal prebiotic fibre intake mitigates some detrimental metabolic outcomes of early overnutrition in rats[J]. European Journal of Nutrition, 2015, 55(8):1-11. DOI:10.1007/s00394-015-1047-2.
[49] LIU T W, CEPHAS K D, HOLSCHER H D, et al. Nondigestible fructans alter gastrointestinal barrier function, gene expression,histomorphology, and the microbiota profiles of diet-induced obese C57BL/6J mice[J]. Journal of Nutrition, 2016, 146(5): 949-956.DOI:10.3945/jn.115.227504.
[50] ZHONG Yadong, NYMAN M, FÅK F. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt[J]. Molecular Nutrition & Food Research, 2015, 59(10): 2066-2076. DOI:10.1002/mnfr.201500187.
[51] SARMA S M, KHARE P, JAGTAP S, et al. Kodo millet whole grain and bran supplementation prevents high-fat diet induced derangements in a lipid prof i le, inf l ammatory status and gut bacteria in mice[J]. Food &Function, 2017, 8(3): 1174-1183. DOI:10.1039/c6fo01467d.
[52] ARIAS M, COBO M, JAIME-SÁNCHEZ P, et al. Gut microbiota and systemic inflammation changes after bread consumption: the ingredients and the processing influence[J]. Journal of Functional Foods, 2017, 32: 98-105. DOI:10.1016/j.jff.2017.02.023.
[53] KEMPERMAN R A, GROSS G, MONDOT S, et al. Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome[J]. Food Research International, 2013, 53(2): 659-669.DOI:10.1016/j.foodres.2013.01.034.
[54] HENNING S M, SUMMANEN P H, LEE R P, et al.Pomegranate ellagitannins stimulate the growth of Akkermansia muciniphila in vivo[J]. Anaerobe, 2016, 43: 56-60. DOI:10.1016/j.anaerobe.2016.12.003.
[55] CHAPLIN A, PARRA P, SERRA F, et al. Conjugated linoleic acid supplementation under a high-fat diet modulates stomach protein expression and intestinal microbiota in adult mice[J]. PLoS ONE,2015, 10(4): e0125091. DOI:10.1371/journal.pone.0125091.
[56] LEAL-DÍAZ A M, NORIEGA L G, TORRE-VILLALVAZO I, et al.Aguamiel concentrate from Agave salmiana and its extracted saponins attenuated obesity and hepatic steatosis and increased Akkermansia muciniphila in C57BL6 mice[J]. Scientif i c Reports, 2016, 6: 34242.DOI:10.1038/srep34242.
[57] SONG Haizhao, CHU Qiang, YAN Fujie, et al. Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice[J].Journal of Gastroenterology & Hepatology, 2015, 31(8): 1462-1469.DOI:10.1111/jgh.13278.
[58] WANG J H, BOSE S, KIM G C, et al. Flos Lonicera ameliorates obesity and associated endotoxemia in rats through modulation of gut permeability and intestinal microbiota[J]. PLoS ONE, 2014, 9(1):e86117. DOI:10.1371/journal.pone.0086117.
[59] WANG Jinghua, BOSE S, KIM H G, et al. Fermented Rhizoma Atractylodis Macrocephalae alleviates high fat diet-induced obesity in association with regulation of intestinal permeability and microbiota in rats[J]. Scientif i c Reports, 2015, 5: 8391. DOI:10.1038/srep08391.
[60] CHANG C J, LIN C S, LU C C, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota[J].Nature Communications, 2015, 6: 7489. DOI:10.1038/ncomms8489.
[61] SHEN Wei, SHEN Mengyu, ZHAO Xia, et al. Anti-obesity effect of capsaicin in mice fed with high-fat diet is associated with an increase in population of the gut bacterium Akkermansia muciniphila[J]. Frontiers in Microbiology, 2017, 8: 272. DOI:10.3389/fmicb.2017.00272.
[62] LIN C H, CHEN Y H, TSAI T Y, et al. Effects of deep sea water and Lactobacillus paracasei subsp. paracasei NTU 101 on hypercholesterolemia hamsters gut microbiota[J]. Applied Microbiology & Biotechnology, 2017, 101(1): 321-329. DOI:10.1007/s00253-016-7868-y.
Recent Advances in the Relationship between Akkermansia muciniphlia and Intestinal Barrier Function
GAO Zhipeng, WU Hua, GENG Xin, SONG Jingyi, ZHANG Kaiqi, XIAO Junsong*
(Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Engineering and Technology Research Center of Food Additives, Beijing Key Laboratory of Plant Resource Research and Development,Beijing Technology and Business University, Beijing 100048, China)
Abstract:Intestinal barrier dysfunction is associated with some chronic metabolic diseases, such as obesity and diabetes.Akkermansia muciniphlia, a mucin-degrading bacterium that resides in the mucus layer, is closely related to intestinal barrier function. Signif i cant changes in the abundance of this bacterium in clinical cases and some animal models of metabolic diseases suggest its possible involvement in the progression of metabolic diseases. However, the exact mechanism of action remains to be clarif i ed. This article reviews recent advances in the relationship of A. muciniphlia with the intestinal mucus layer, metabolic diseases and intestinal immunity, and also attempts to elucidate the inf l uence and mechanism of action of this bacterium on intestinal barrier function. It is hoped that this review will provide new insights into dietary prevention of chronic metabolic diseases.
Keywords:Akkermansia muciniphlia; intestinal barrier function; chronic metabolic diseases; abundance
GAO Zhipeng, WU Hua, GENG Xin, et al. Recent advances in the relationship between Akkermansia muciniphlia and intestinal barrier function[J]. Food Science, 2018, 39(19): 296-302. (in Chinese with English abstract) DOI:10.7506/spkx1002-6630-201819045. http://www.spkx.net.cn
高志鹏, 吴华, 耿欣, 等. 肠道黏液层降解菌Akkermansia muciniphlia和肠道屏障功能关系研究进展[J]. 食品科学, 2018,39(19): 296-302. DOI:10.7506/spkx1002-6630-201819045. http://www.spkx.net.cn
引文格式:
文章编号:1002-6630(2018)19-0296-07
文献标志码:A
中图分类号:TS201.3
DOI:10.7506/spkx1002-6630-201819045
*通信作者简介:肖俊松(1980—),男,副教授,博士,研究方向为功能性食品与肠道菌群。E-mail:xiaojs@th.btbu.edu.cn
第一作者简介:高志鹏(1994—),男,硕士研究生,研究方向为肠道菌群。E-mail:wooden_cheat@qq.com
北京工商大学两科基金培育项目(LKJJ2016-18)
基金项目:北京市自然科学基金项目(7162028);“十三五”国家重点研发计划重点专项(2016YFD0400801);
收稿日期:2017-09-06