表1 模拟汁中不同质量浓度的可同化氮和还原糖试验设计
Table 1 Different combinations of YAN and reducing sugar in synthetic grape must

姜 越1,潘 婷1,惠竹梅1,2,*
(1.西北农林科技大学葡萄酒学院,陕西 杨凌 712100;2.陕西葡萄与葡萄酒工程中心,陕西 杨凌 712100)
摘 要:为研究葡萄汁中可同化氮和还原糖对酵母发酵特性的影响,设计150、240、330、420、500 mg/L可同化氮质量浓度和170、200、230 g/L还原糖质量浓度,共计15 个处理,测定了模拟葡萄汁酒精发酵过程中酵母生长、还原糖消耗和可同化氮消耗的变化。结果表明,模拟汁中可同化氮质量浓度过低(150 mg/L)则不能充分满足酵母生长的需要,同时限制了酵母的还原糖消耗速率,通过提高初始还原糖质量浓度至200 g/L可促进酒精发酵进行;酵母在初始可同化氮质量浓度高于240 mg/L的模拟汁中可以正常生长,此时初始还原糖、可同化氮质量浓度对酵母生长量均无显著影响,还原糖含量最直接影响酿酒酵母菌株的发酵特性,决定发酵时间长短,表现为在初始还原糖质量浓度较低(170 g/L)的模拟汁中,酵母生长速率随着模拟汁初始可同化氮质量浓度的升高而加快,在初始还原糖质量浓度较高(200~230 g/L)的模拟汁中,酵母生长速率不受初始可同化氮质量浓度的影响;当模拟汁初始可同化氮质量浓度高于330 mg/L时,酵母对可同化氮的消耗开始出现剩余,剩余量随着模拟汁初始可同化氮质量浓度的升高而增加,此时可同化氮质量浓度能够充分满足酵母可同化氮代谢的需要,且酵母对可同化氮消耗量随着初始还原糖质量浓度的增加而略有减少。
关键词:模拟葡萄汁;可同化氮;还原糖;酿酒酵母;发酵特性
葡萄汁中含有多种含氮的分子,包括无机氮和有机氮,但只有铵态氮、游离α-氨基酸(脯氨酸除外)和小分子多肽能够被酵母同化,这类氮被称为可同化氮[1-3]。可同化氮是酵母酒精发酵最重要的营养物质,可同化氮缺乏会影响酵母细胞的生长和发酵效率,最终影响葡萄酒中风味代谢物的形成,可同化氮的缺乏还易使葡萄酒发生微生物腐败[4-7]。葡萄汁中可同化氮质量浓度一般在60~500 mg/L(以N计),酿造葡萄酒时,对于可同化氮质量浓度低于150 mg/L的葡萄汁,通常认为酵母在发酵中会受到氮源限制,因此会添加氮源以防止因氮素缺乏对酵母酒精发酵带来的不利影响[3,8]。
葡萄汁中的糖对酵母来说非常重要,酵母发酵其他底物的能力有限,最重要的营养物质和新陈代谢所需能量大部分来自糖,糖为酵母的生长代谢提供能量和构建细胞组成的碳骨架,直接或间接调节着所有主要的代谢途径,这些代谢活动会影响酵母的酒精发酵,并最终导致包括酸类、酯类、高级醇、羰基化合物和硫醇等影响葡萄酒香气的副产物产生[2,9-13]。
目前,国内外学者关于葡萄酒酿造中可同化氮对酵母酒精发酵的影响研究较多,许多研究结果表明葡萄汁中可同化氮水平会影响酵母的酒精发酵[14-17],但关于葡萄汁中可同化氮和还原糖两者对酵母酒精发酵的影响研究很少,本研究以模拟葡萄汁为试材,探索不同还原糖质量浓度对酵母利用可同化氮素能力的影响,寻找出有利于酵母酒精发酵的可同化氮和还原糖质量浓度,为不同含糖量的葡萄汁中添加可同化氮素提供一定的理论和参考依据。
酿酒酵母Lalvin EC1118来自法国Laffort公司,性状稳定优良。
谷氨酰胺、色氨酸、苏氨酸、组氨酸和亮氨酸等(均为分析纯) 美国Sigma公司;葡萄糖、苹果酸、柠檬酸、酒石酸、肌醇、泛酸钙、烟酸、盐酸硫胺、盐酸吡哆醇、生物素和磷酸氢二铵等均为国产分析纯。
UV-1800分光光度计 日本Shimadzu公司;Centrifuge 5804R离心机 德国Eppendorf公司;Rocker 300无油真空泵 台湾洛科仪器股份有限公司;7890/5975B气相色谱-质谱仪 美国安捷伦公司。
1.3.1 试验设计
实验在西北农林科技大学葡萄酒学院实验室进行,研究不同质量浓度可同化氮和还原糖两个因素对模拟葡萄汁酒精发酵的影响,共设15个处理,具体设计见表1。
表1 模拟汁中不同质量浓度的可同化氮和还原糖试验设计
Table 1 Different combinations of YAN and reducing sugar in synthetic grape must

1.3.2 模拟葡萄汁的配制
模拟葡萄汁根据Beltran[18]和Riou[19]等的方法配制。模拟汁分别由储液Ⅰ、储液Ⅱ、储液Ⅲ和储液Ⅳ组成,储液Ⅰ为可同化氮储液,储液Ⅱ为葡萄糖储液,储液Ⅲ为有机酸储液,储液Ⅳ为矿物盐和维生素储液。将储液Ⅰ、Ⅱ、Ⅲ和Ⅳ混合后用NaOH调节pH值至3.3,0.45 μm滤膜过滤除菌,现用现配。
1.3.3 指标测定
模拟汁在半厌氧条件下进行发酵,发酵期间(每隔24 h)及发酵结束后分别取样进行各项指标测定。
1.3.3.1 发酵过程中各指标的测定
酵母生长量利用分光光度法在600 nm波长处测定;还原糖采用斐林试剂热滴定法测定[20];测定可同化氮前取发酵液于11 000 r/min离心5 min,取上清液,测定时采用甲醛滴定法[21]。
1.3.3.2 发酵后基本指标的测定
总酸和挥发酸的含量测定采用NaOH滴定法[20];酒精度的测定采用比重瓶法[20]。
数据采用Excel 2010数据处理软件进行分析处理,差异显著性分析采用Duncan新复极差法。
2.1.1 模拟汁中可同化氮质量浓度对酵母生长的影响
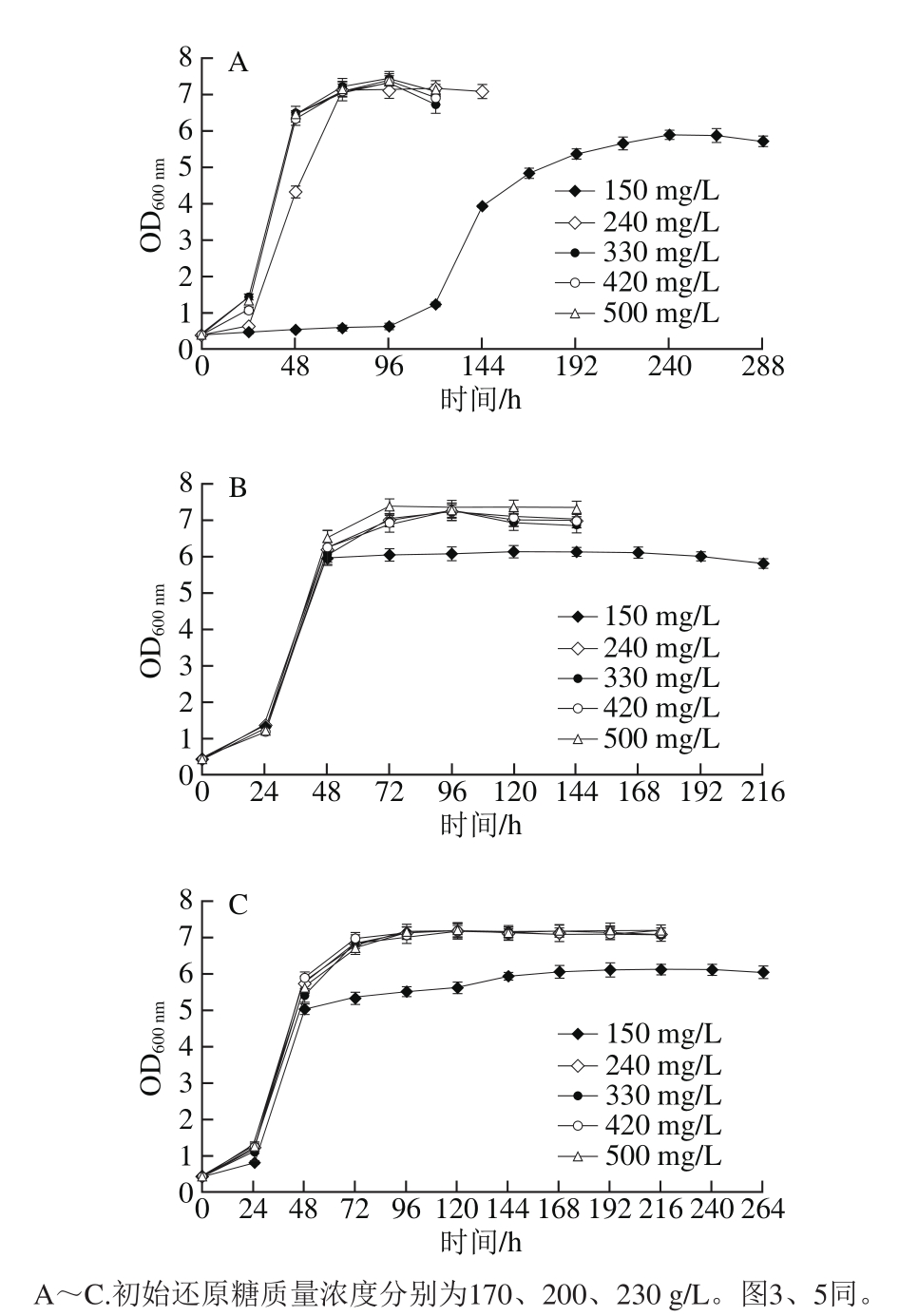
图1 可同化氮质量浓度对模拟汁发酵中酵母生长的影响
Fig. 1 Effect of different YAN concentrations on the growth of S. cerevisiae
由图1可知,在3 种还原糖质量浓度下,初始可同化氮质量浓度为150 mg/L的模拟汁中,酵母生长量在生长平稳期均显著低于可同化氮质量浓度为240~500 mg/L的模拟汁(P<0.05),且发酵时间最长,此时240~500 mg/L 4 种可同化氮质量浓度的模拟汁中酵母生长量无显著差异。在初始还原糖质量浓度为170 g/L的模拟汁中,酵母生长速率随着模拟汁初始可同化氮质量浓度的升高而加快,其中初始可同化氮质量浓度为150 mg/L的模拟汁中酵母在发酵初期存在生长停滞的风险;而在初始还原糖质量浓度为200~230 g/L的模拟汁中,处于对数生长期的酵母生长速率不受初始可同化氮质量浓度的影响。说明150 mg/L可同化氮质量浓度过低,不能充分满足酵母生长的需要;初始可同化氮质量浓度为240 mg/L已经能完全满足酵母生长需要;高于此质量浓度时,可同化氮虽然会提高低糖模拟汁中酵母初期发酵速率,但对酵母生长量无明显影响,这与Beltran[22]和Vilanova[23]等的研究一致。可同化氮作为酵母生长的必须营养元素,为酵母蛋白质和核苷酸的合成提供前体物质,较高质量浓度可同化氮能够提高酵母生长量和代谢活性,刺激发酵活动[24-27]。
2.1.2 模拟汁中还原糖质量浓度对酵母生长的影响
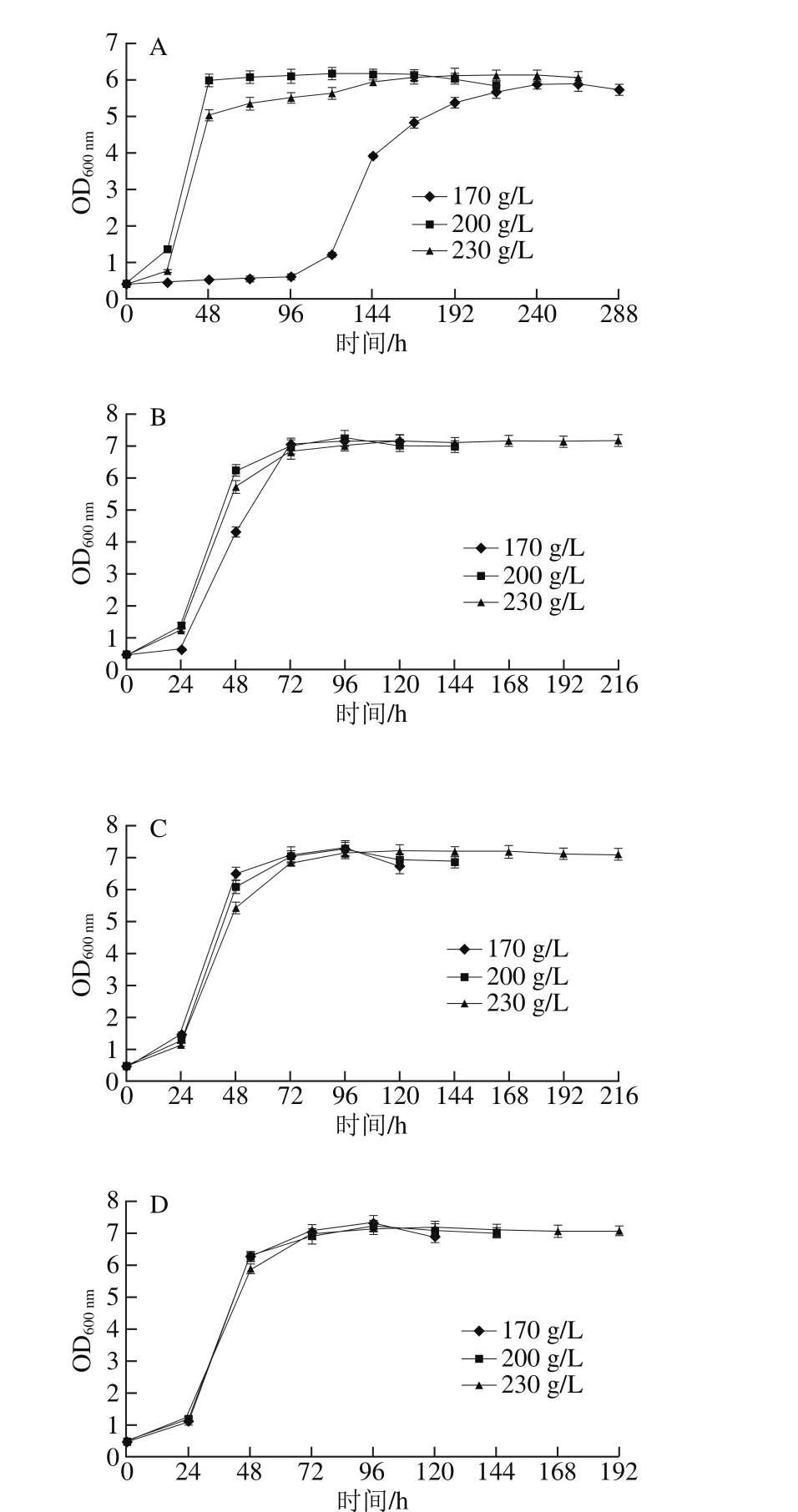
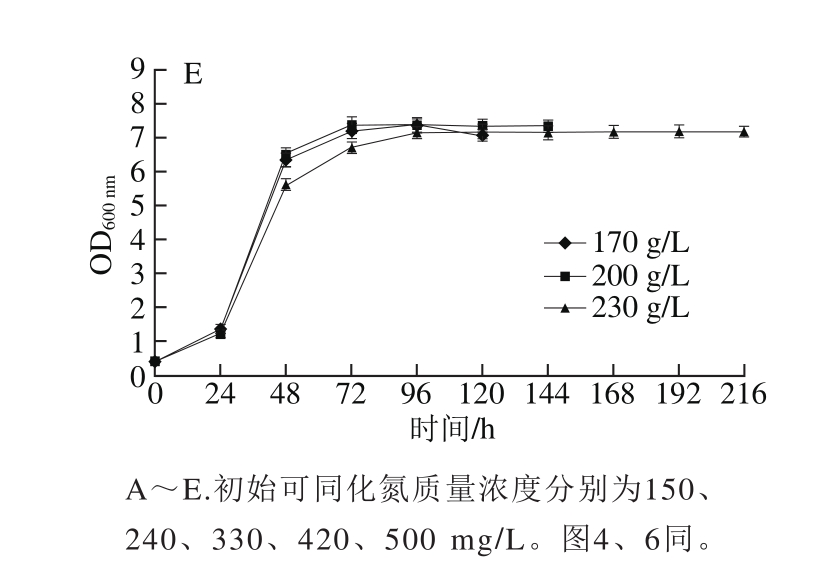
图2 还原糖质量浓度对模拟汁发酵中酵母生长的影响
Fig. 2 Effect of different reducing sugar concentrations on the growth of S. cerevisiae
由图2可知,当初始可同化氮质量浓度较低(150~240 mg/L)时,在还原糖质量浓度为170 g/L的模拟汁中,酵母在对数生长期的生长速率显著低于200 g/L和230 g/L两种还原糖质量浓度的模拟汁,甚至在初始可同化氮质量浓度为150 mg/L、初始还原糖质量浓度为170 g/L的模拟汁中酵母出现发酵初期生长停滞的现象,说明在可同化氮缺乏的模拟汁中可以通过适量提高还原糖质量浓度的方法促进发酵进行;当初始可同化氮质量浓度较高(330~500 mg/L)时,还原糖质量浓度为230 g/L的模拟汁中酵母对数生长期生长速率低于其他两种初始还原糖质量浓度的模拟汁,说明还原糖和可同化氮质量浓度同时过高或过低均不利于酵母生长。在相同初始可同化氮质量浓度的模拟汁中,3 种还原糖质量浓度下的酵母在平稳期时生长量均无显著性差异,均可完成发酵过程,说明在模拟汁中可同化氮源充足的情况下,初始还原糖质量浓度对酵母生长量无明显影响。
2.2.1 模拟汁中可同化氮质量浓度对酵母还原糖消耗的影响
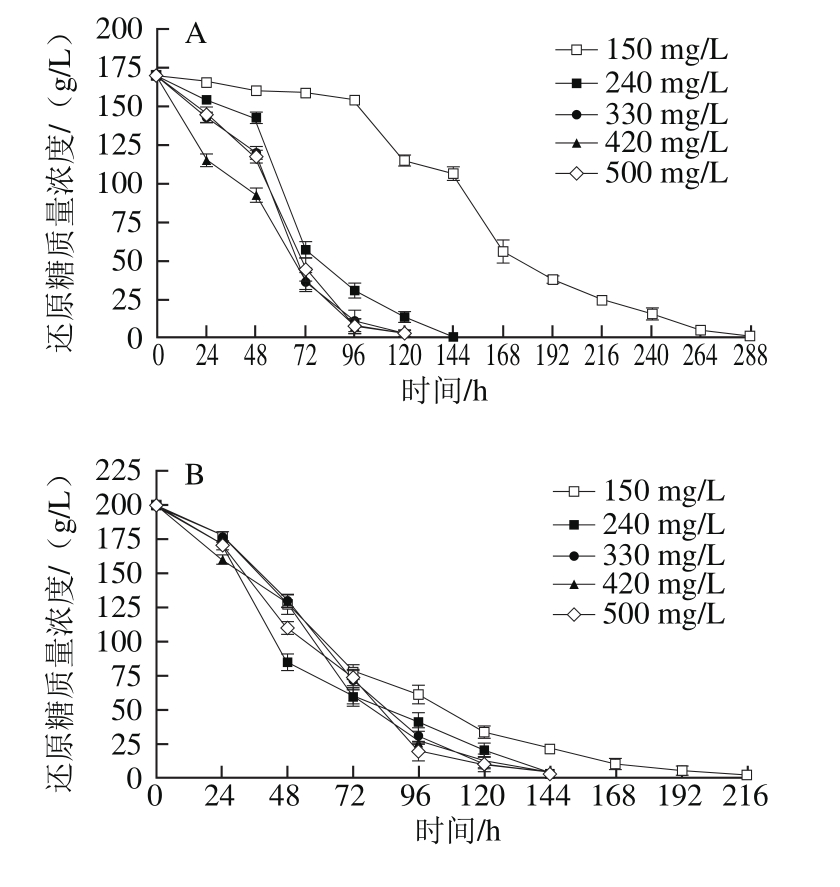
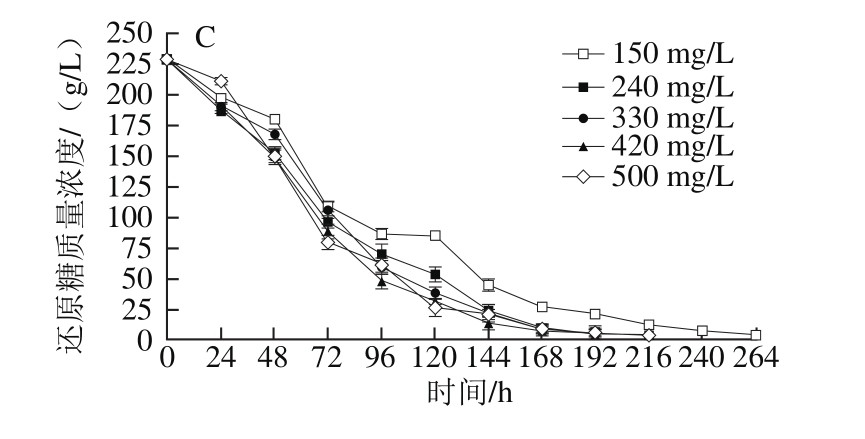
图3 可同化氮质量浓度对模拟汁发酵中还原糖消耗的影响
Fig. 3 Effect of different YAN concentrations on reducing sugar consumption
由图3可知,在发酵过程中,酵母对还原糖的消耗速率表现为先快后慢。在3 种初始还原糖质量浓度的模拟汁中,酵母在可同化氮质量浓度为150 mg/L的模拟汁中完成酒精发酵的时间均最长,随着初始可同化氮质量浓度升高,发酵时间明显缩短,说明150 mg/L可同化氮质量浓度过低,限制了酵母的还原糖消耗速率,提高模拟汁可同化氮质量浓度能够促进酵母的糖代谢过程[23,28-29]。也有研究表明,在没有其他生长和发酵限制因素存在的情况下,葡萄汁中的可同化氮质量浓度在很大程度上决定了酵母的发酵速率[2,17]。Torrea等[30]研究表明,在160 mg/L可同化氮质量浓度的葡萄汁中,酵母完成发酵所需时间为13 d,中等可同化氮质量浓度时(320 mg/L)减少为7 d,高可同化氮质量浓度(480 mg/L)时只需5 d。
2.2.2 模拟汁中还原糖质量浓度对酵母还原糖消耗的影响
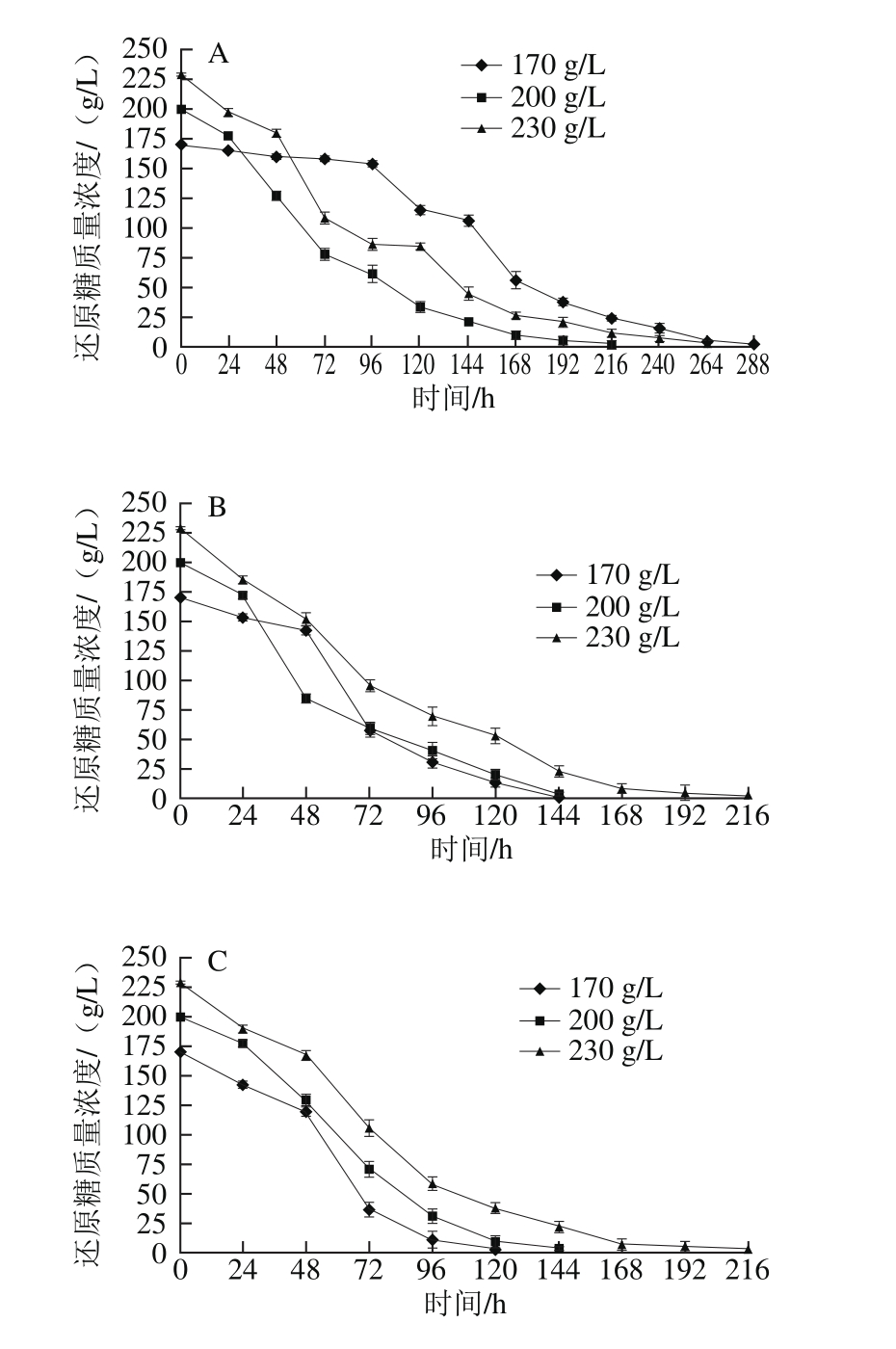
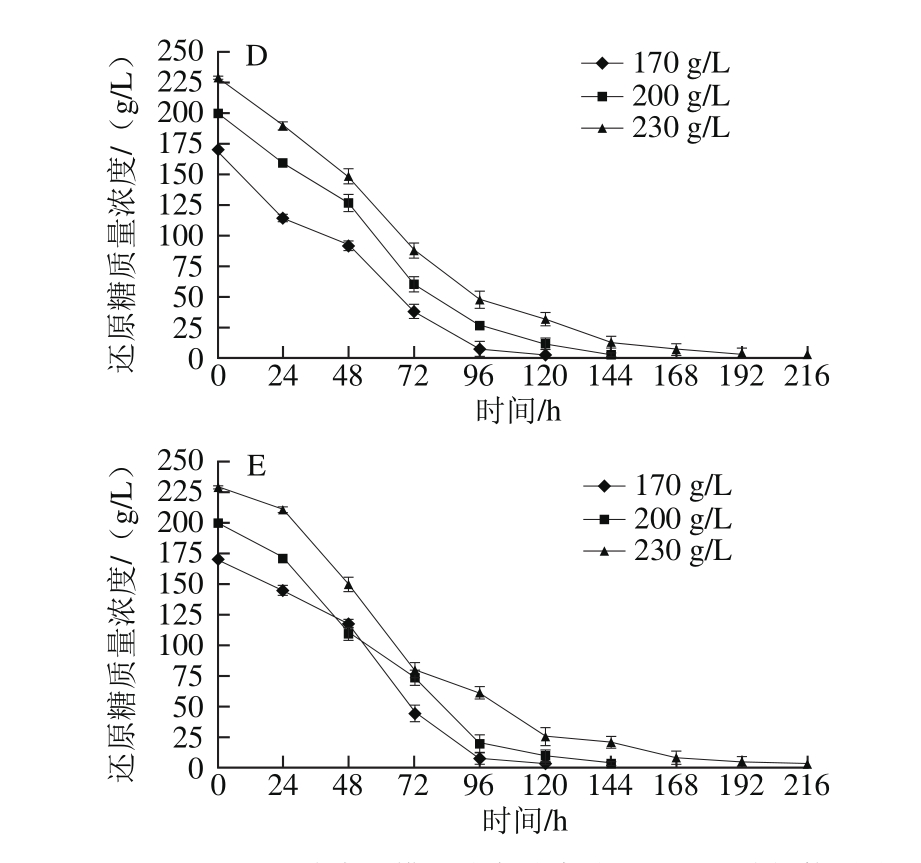
图4 还原糖质量浓度对模拟汁发酵中酵母还原糖消耗的影响
Fig. 4 Effect of different reducing sugar concentrations on sugar consumption
由图4可知,当初始可同化氮质量浓度为150 mg/L时,在还原糖质量浓度为170 g/L的模拟汁中,酵母在发酵初期出现还原糖消耗停滞,而酵母在其他两种还原糖质量浓度中糖消耗正常,说明当模拟汁中可同化氮质量浓度低时,酵母需要较高的初始还原糖质量浓度才能进行正常的酒精发酵;在240~500 mg/L 4 种初始可同化氮质量浓度的模拟汁中,酵母在相同可同化氮质量浓度下完成酒精发酵时间基本均随还原糖质量浓度升高而延长,说明在正常的酒精发酵中,在可同化氮充足时,还原糖作为葡萄汁发酵的主要底物,对酿酒酵母菌株的发酵特性有着最直接的影响,决定了发酵时间的长短[31]。
2.3.1 模拟汁中可同化氮质量浓度对酵母可同化氮消耗的影响
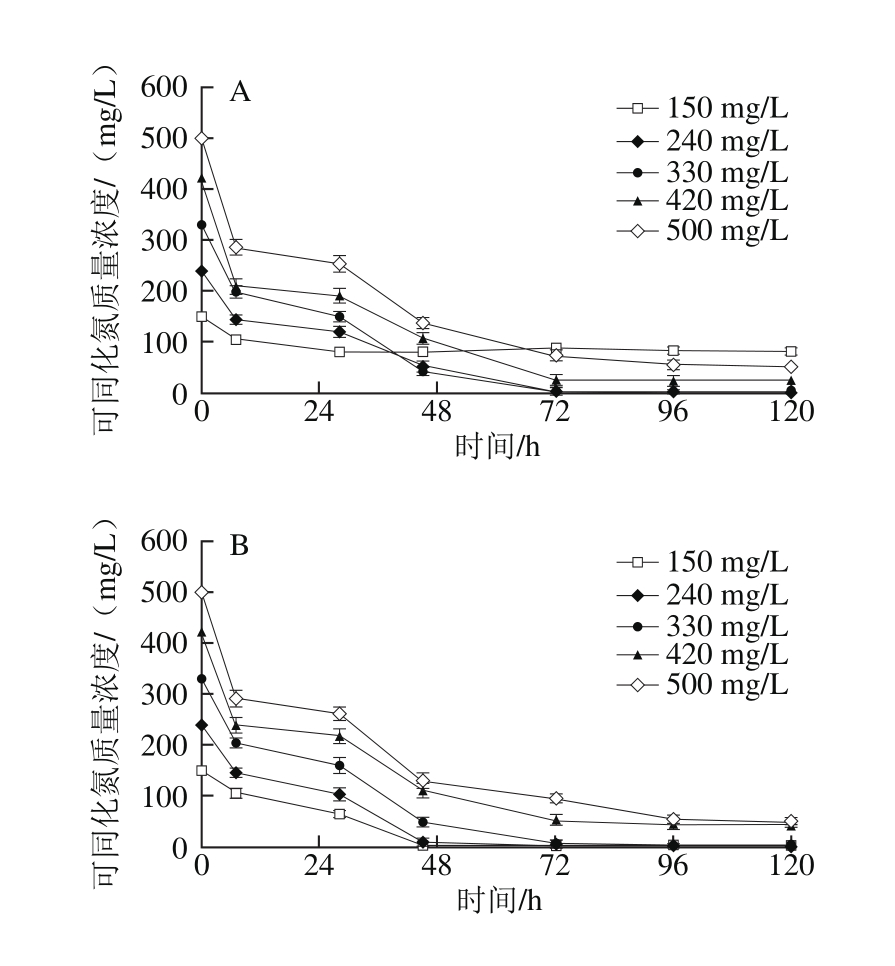

图5 可同化氮质量浓度对模拟汁发酵中可同化氮消耗的影响
Fig. 5 Effect of different YAN concentrations on nitrogen consumption
由图5可知,在初始还原糖质量浓度为170 g/L的模拟汁中,当初始可同化氮质量浓度为150 mg/L时,可同化氮消耗率小于50%,可同化氮消耗异常。在可同化氮正常消耗的14 种模拟汁中,酵母在发酵0~7 h和发酵28~45 h可同化氮消耗迅速,在发酵7~28 h和45~72 h可同化氮消耗缓慢;当初始可同化氮质量浓度为150~330 mg/L时,可同化氮均在酒精发酵72 h内被完全消耗;当初始可同化氮质量浓度为420~500 mg/L时,3 种还原糖质量浓度的模拟汁在发酵完成时均出现可同化氮剩余,剩余量随着模拟汁初始可同化氮质量浓度的升高而增加,并在酒精发酵72 h后基本保持稳定,Mendes-Ferreira[22]和Beran[28]等研究表明酵母在模拟汁发酵中消耗掉全部可同化氮时间为48~72 h,与本实验研究结果相似,说明酵母在发酵初期对可同化氮的利用率较高,且420~500 mg/L可同化氮质量浓度能够充分满足酵母可同化氮代谢的需要。
2.3.2 模拟汁中还原糖质量浓度对酵母可同化氮消耗的影响
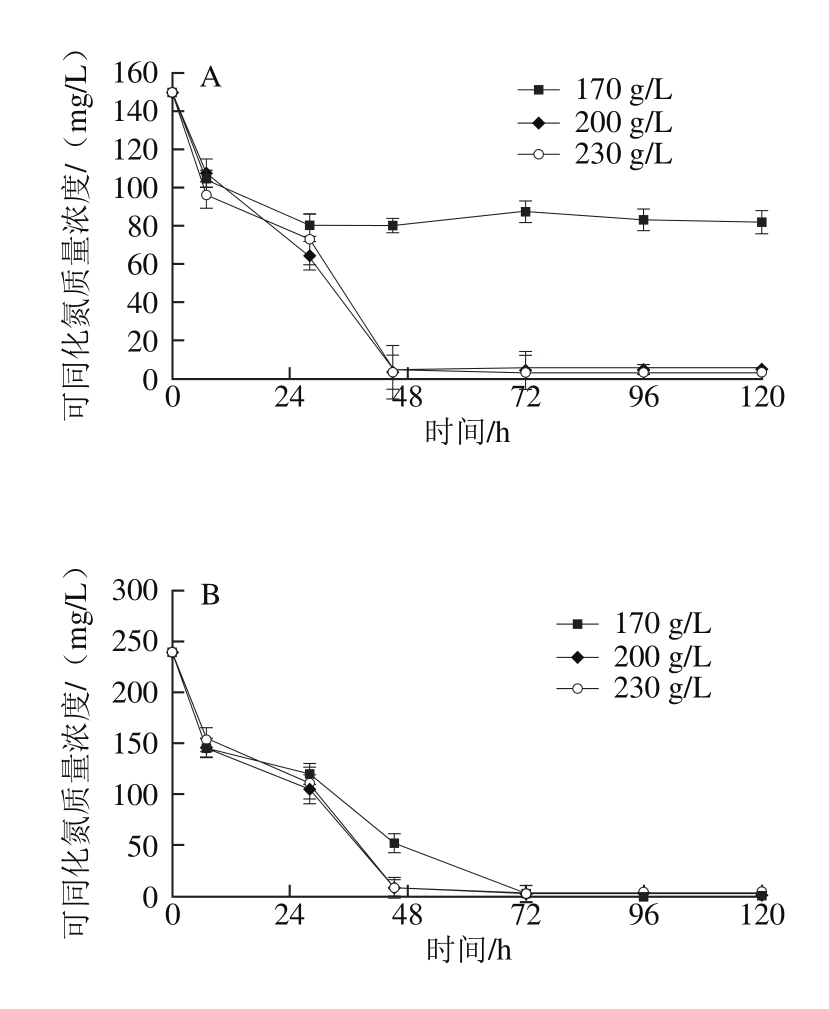

图6 还原糖质量浓度对模拟汁发酵中酵母可同化氮消耗的影响
Fig. 6 Effect of different reducing sugar concentrations on nitrogen consumption
由图6可知,当初始可同化氮质量浓度为150 mg/L时,在初始还原糖质量浓度为170 g/L的模拟汁中,可同化氮的消耗量远小于其他两个还原糖质量浓度(P<0.05);在其余14 种模拟汁中,当初始可同化氮质量浓度较低(150~240 mg/L)时,模拟汁中初始还原糖质量浓度对酵母在发酵中可同化氮消耗量的影响不明显;当初始可同化氮质量浓度较高(330~500 mg/L)时,可同化氮剩余量随着模拟汁中初始还原糖质量浓度的增加而升高。Jiranek等[32]研究发现在酒精发酵中酵母对可同化氮的需求量与葡萄汁中还原糖质量浓度有关,随着初始还原糖质量浓度的升高,不同的酵母菌株对可同化氮的利用呈增加或减少的趋势。在本实验中,当模拟汁中初始可同化氮充足(330~500 mg/L)时,初始还原糖质量浓度对酵母可同化氮的利用有减少的趋势,但可同化氮的消耗量无显著性差异。
由表2可知,各处理中酒样的基本指标均达GB/T 15038—2006《葡萄酒、果酒通用分析方法》要求,模拟酒的pH值在3.15~3.36之间,残糖不大于4 g/L。当模拟汁中初始还原糖质量浓度相同时,发酵后模拟酒中的总酸随初始可同化氮质量浓度的升高而增加,当模拟汁中初始可同化氮质量浓度相同时,除240 mg/L可同化氮质量浓度外,其他可同化氮质量浓度的模拟汁发酵后的总酸均随初始还原糖质量浓度升高而增加。另外,模拟酒中的酒精度随还原糖质量浓度的升高而增加。
表2 发酵后各模拟酒基本指标
Table 2 Quality parameters of fermented synthetic grape musts
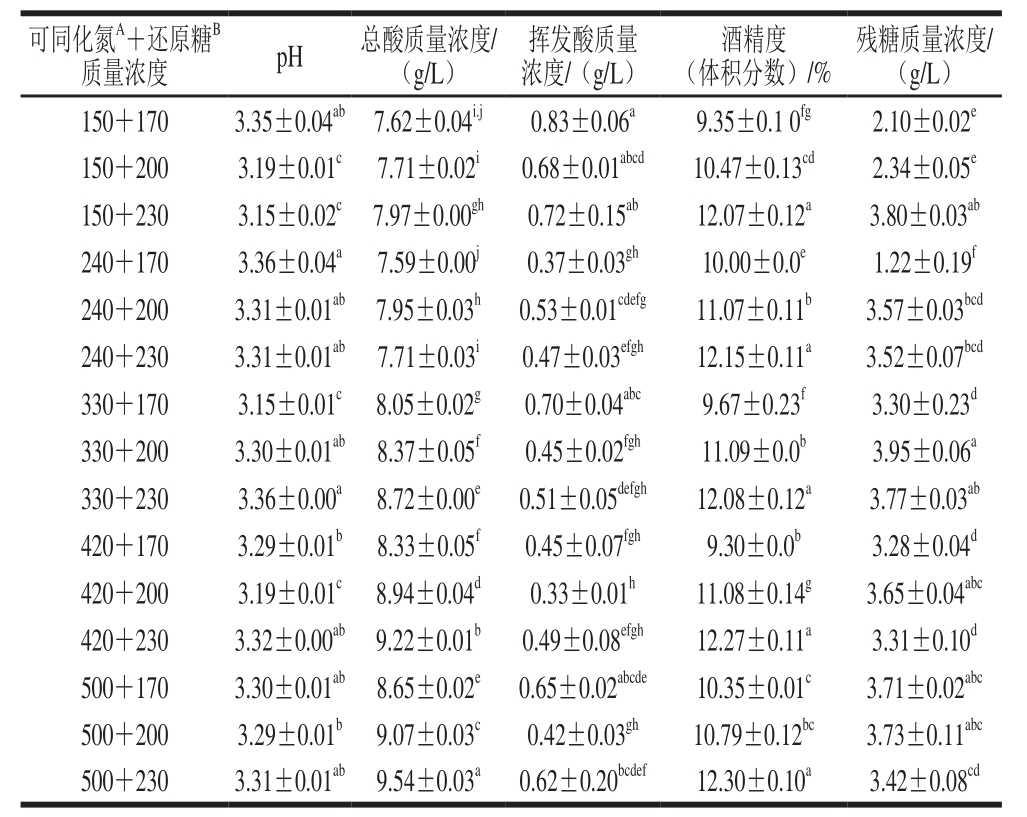
注:同列肩标小写字母不同表示差异显著(P<0.05)。肩标A.单位为mg/L;B.单位为g/L。
本实验对不同初始可同化氮和还原糖质量浓度的模拟汁中酵母生长量、还原糖和可同化氮消耗量等发酵特性进行了比较分析,结果表明:当初始可同化氮质量浓度为150 mg/L、还原糖质量浓度为170 g/L时,酵母在发酵初期生长停滞,不能保证正常的酒精发酵,通过提高模拟汁中初始还原糖质量浓度或者可同化氮质量浓度可以防止此类问题出现;当可同化氮质量浓度为150 mg/L时,在3 种还原糖质量浓度的模拟汁中,酵母的生长和还原糖消耗速率均受到限制,提高可同化氮质量浓度可以显著促进酵母生长和酒精发酵(P<0.05);330~500 mg/L可同化氮质量浓度能充分满足酵母酒精发酵的需要,此时氮源充足,初始还原糖质量浓度对酵母生长量无明显影响,且发酵结束后可同化氮剩余量随初始可同化氮质量浓度升高而增加。
参考文献:
[1] 惠竹梅, 吕万祥, 刘延琳. 可同化氮素对葡萄酒发酵香气影响研究进展[J]. 中国农业科学, 2011, 44(24): 5058-5066. DOI:10.3864/j.issn.0578-1752.2011.24.011.
[2] INGLEDEW W M, MAGNUS C A, SOSULSKI F W. Influence of oxygen on proline utilization during the wine fermentation[J].American Journal of Enology and Viticulture, 1987, 38(3): 246-248.
[3] BELL S, HENSCHKE P A. Implications of nitrogen nutrition for grapes, fermentation and wine[J]. Australian Journal of Grape and Wine Research, 2005, 11(3): 242-295. DOI:10.1111/j.1755-0238.2005.tb00028.x.
[4] JÚNIOR M M, BATISTOTE M, ERNANDES J R. Glucose and fructose fermentation by wine yeasts in media containing structurally complex nitrogen sources[J]. Journal of the Institute of Brewing, 2008,114(3): 199-204.
[5] BACH B, COLAS S, MASSINI L, et al. Effect of nitrogen addition during alcoholic fermentation on the final content of biogenic amines in wine[J]. Annals of Microbiology, 2011, 61(1): 185-190.DOI:10.1007/s13213-010-0119-z.
[6] JIMÉNEZ-MARTÍ E, ARANDA A, MENDES-FERREIRA A, et al. The nature of the nitrogen source added to nitrogen depleted vinifications conducted by a Saccharomyces cerevisiae strain in synthetic must affects gene expression and the levels of several volatile compounds[J]. Antonie Van Leeuwenhoek, 2007, 92(1):61-75. DOI:10.1007/s10482-006-9135-1.
[7] HANNAM K D, NEILSEN G H, FORGE T, et al. The concentration of yeast assimilable nitrogen in Merlot grape juice is increased by N fertilization and reduced irrigation[J]. Canadian Journal of Plant Science, 2013, 93(1): 37-45. DOI:10.4141/CJPS2012-092.
[8] BRICE C, SANCHEZ I, TESNIÈRE C, et al. Assessing the mechanisms responsible for differences between nitrogen requirements of Saccharomyces cerevisiae wine yeasts in alcoholic fermentation[J].Applied and Environmental Microbiology, 2014, 80(4): 1330-1339.DOI:10.1128/AEM.03856-13.
[9] JIRANEK V, LANGRIDGE P, HENSCHKE P A, et al. Amino acid and ammonium utilization by Saccharomyces cerevisiae wine yeasts from a chemically defined medium[J]. Applied and Environmental Microbiology, 1995, 46(1): 75-82.
[10] BAUER F F, PRETORIUS I S. Yeast stress response and fermentation efficiency: how to survive the making of wine: a review[J]. South African Journal for Enology and Viticulture, 2000, 21: 27-51.
[11] SWIEGERS J H, BARTOWSKY E J, HENSCHKE P A, et al. Yeast and bacterial modulation of wine aroma and flavour[J]. Australian Journal of Grape and Wine Research, 2005, 11(2): 139-173.DOI:10.1111/j.1755-0238.2005.tb00285.x.
[12] JACKSON R S. Wine science: principles and applications[M].Academic Press, 2008.
[13] ARROYO-LOPEZ F N, ORLIC S, QUEROL A, et al. Effects of temperature, pH and sugar concentration on the growth parameters of Saccharomyces cerevisiae, S. kudriavzevii and their interspecific hybrid[J]. International Journal of Food Microbiology, 2009, 131(2):120-127. DOI:10.1016/j.ijfoodmicro.2009.01.035.
[14] D’AMATO D, CORBO M R, NOBILE M A D, et al. Effects of temperature, ammonium and glucose concentrations on yeast growth in a model wine system[J]. International Journal of Food Science &Technology, 2006, 41(10): 1152-1157. DOI:10.1111/j.1365-2621.2005.01128.x.
[15] CARRAU F M, MEDINA K, FARINA L, et al. Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: effects of yeast assimilable nitrogen on two model strains[J].FEMS Yeast Research, 2008, 8(7): 1196-1207. DOI:10.1111/j.1567-1364.2008.00412.x.
[16] HERNÁNDEZ-ORTE P, IBARZ M J, CACHO J, et al. Effect of the addition of ammonium and amino acids to musts of Airen variety on aromatic composition and sensory properties of the obtained wine[J]. Food Chemistry, 2005, 89(2): 163-174. DOI:10.1016/j.foodchem.2004.02.021.
[17] MARTÍNEZ-MORENO R, MORALES P, GONZALEZ R, et al. Biomass production and alcoholic fermentation performance of Saccharomyces cerevisiae as a function of nitrogen source[J].FEMS Yeast Research, 2012, 12(4): 477-485. DOI:10.1111/j.1567-1364.2012.00802.x.
[18] BELTRAN G, ESTEVE-ZARZOSO B, ROZES N, et al. Influence of the timing of nitrogen additions during synthetic grape must fermentations on fermentation kinetics and nitrogen consumption[J].Journal of Agricultural and Food Chemistry, 2005, 53(4): 996-1002.DOI:10.1021/jf0487001.
[19] RIOU C, NICAUD J M, BARRE P, et al. Stationary-phase gene expression in Saccharomyces cerevisiae during wine fermentation[J].Yeast, 1997, 13(10): 903-915. DOI:10.1002/(SICI)1097-0061(199708)13:10<903::AID-YEA145>3.3.CO;2-T.
[20] 王华. 葡萄酒分析检测[M]. 杨凌: 西北农林科技大学, 2004.
[21] GUMP B H, ZOECKLEIN B W, FUGELSANG K C. Prediction of prefermentation nutritional status of grape juice[M]//Food Microbiology Protocols. Humana Press, 2001: 283-296.
[22] BELTRAN G, NOVO M, ROZES N, et al. Nitrogen catabolite repression in Saccharomyces cerevisiae during wine fermentation[J].FEMS Yeast Research, 2004, 4(6): 625-632. DOI:10.1016/j.femsyr.2003.12.004.
[23] VILANOVA M, UGLIANO M, VARELA C, et al. Assimilable nitrogen utilisation and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts[J]. Applied Microbiology and Biotechnology,2007, 77(1): 145-157. DOI:10.1007/s00253-007-1145-z.
[24] HALLINAN C P, SAUL D J, JIRANEK V. Differential utilisation of sulfur compounds for H2S liberation by nitrogen-starved wine yeasts[J]. Australian Journal of Grape and Wine Research, 1999, 5(3):82-90. DOI:10.1111/j.1755-0238.1999.tb00291.x.
[25] BISSON L F, BUTZKE C E. Diagnosis and rectification of stuck and sluggish fermentations[J]. American Journal of Enology and Viticulture, 2000, 51(2): 168-177.
[26] SPIROPOULOS A, TANAKA J, FLERIANOS I, et al.Characterization of hydrogen sulfide formation in commercial and natural wine isolates of Saccharomyces[J]. American Journal of Enology and Viticulture, 2000, 51(3): 233-248.
[27] CASALTA E, CERVI M F, SALMON J M, et al. White wine fermentation: interaction of assimilable nitrogen and grape solids[J].Australian Journal of Grape and Wine Research, 2013, 19(1): 47-52.DOI:10.1111/j.1755-0238.2012.00205.x.
[28] MENDES-FERREIRA A, MENDES-FAIA A, LEÃO C. Growth and fermentation patterns of Saccharomyces cerevisiae under different ammonium concentrations and its implications in winemaking industry[J]. Journal of Applied Microbiology, 2004, 97(3): 540-545.DOI:10.1111/j.1365-2672.2004.02331.x.
[29] VARELA C, PIZARRO F, AGOSIN E. Biomass content governs fermentation rate in nitrogen-def i cient wine musts[J]. Applied and Environmental Microbiology, 2004, 70(6): 3392-3400. DOI:10.1128/AEM.70.6.3392-3400.2004.
[30] TORREA D, VARELA C, UGLIANO M, et al. Comparison of inorganic and organic nitrogen supplementation of grape juice: effect on volatile composition and aroma profile of a Chardonnay wine fermented with Saccharomyces cerevisiae yeast[J]. Food Chemistry,2011, 127(3): 1072-1083. DOI:10.1016/j.foodchem.2011.01.092.
[31] LEGODI L M. Improving wine yeast for fructose and nitrogen utilization[D]. Cape Town: Stellenbosch University, 2008.
[32] JIRANEK V, LANGRIDGE P, HENSCHKE P A. Regulation of hydrogen sulfide liberation in wine-producing Saccharomyces cerevisiae strains by assimilable nitrogen[J]. Applied and Environmental Microbiology, 1995, 61(2): 461-467.
Effect of Assimilable Nitrogen and Reducing Sugar Concentrations of Synthetic Grape Must on the Fermentation Characteristics of Saccharomyces cerevisiae
JIANG Yue1, PAN Ting1, XI Zhumei1,2,*
(1. College of Enology, Northwest A&F University, Yangling 712100, China;2. Shaanxi Engineering Research Center for Viti-Viniculture, Yangling 712100, China)
Abstract:In this study, fifteen combination treatments were designed using five yeast assimilable nitrogen (YAN)concentrations (150, 240, 330, 420, and 500 mg/L) and three reducing sugar concentrations (170, 200, and 230 g/L) to study the effects of different concentrations of assimilable nitrogen and reducing sugar in synthetic grape must on the fermentation characteristics of Saccharomyces cerevisiae. For this purpose, yeast growth, sugar consumption rate and nitrogen consumption were measured. The results showed that 150 mg/L of YAN nitrogen in the synthetic grape must was too low to support yeast growth and simultaneously restricted the consumption rate of reducing sugar by yeast. The rate of alcoholic fermentation was increased by increasing the initial concentration of reducing sugar to 200 g/L. Yeast could grow normally in the synthetic grape must with initial assimilable nitrogen concentrations of higher than 240 mg/L. In this case, initial reducing sugar and assimilable nitrogen concentration had no signif i cant effect on yeast growth, and reducing sugar concentration had the most direct effect on the fermentation characteristics of S. cerevisiae strains, determining the fermentation time. In the synthetic grape must with low initial reducing sugar concentration (170 g/L), the yeast growth rate increased with increasing the initial assimilable nitrogen concentration, while at high initial reducing sugar concentration(200–230 g/L), the yeast growth rate was not affected by the initial assimilable nitrogen concentration. An initial assimilable nitrogen concentration of higher than 330 mg/L was not completely consumed, and the remaining amount increased with the increase in the initial concentration of assimilable nitrogen, providing enough assimilable nitrogen for yeast to grow.Moreover, yeast could decrease assimilable nitrogen consumption with increasing the initial reducing sugar concentration.
Keywords:synthetic grape must; yeast assimilable nitrogen; reducing sugar; Saccharomyces cerevisiae; fermentation characteristics
DOI:10.7506/spkx1002-6630-201802021
中图分类号:TS261.2
文献标志码:A
文章编号:1002-6630(2018)02-0131-07
引文格式:姜越, 潘婷, 惠竹梅. 模拟葡萄汁中可同化氮和还原糖对酵母发酵特性的影响[J]. 食品科学, 2018, 39(2): 131-137.
DOI:10.7506/spkx1002-6630-201802021. http://www.spkx.net.cn
JIANG Yue, PAN Ting, XI Zhumei. Effect of assimilable nitrogen and reducing sugar concentrations of synthetic grape must on the fermentation characteristics of Saccharomyces cerevisiae[J]. Food Science, 2018, 39(2): 131-137. (in Chinese with English abstract)
DOI:10.7506/spkx1002-6630-201802021. http://www.spkx.net.cn
收稿日期:2017-02-14
基金项目:国家现代农业产业技术体系建设专项(CARS-30);陕西省果业局专项(K332021412)
第一作者简介:姜越(1993—),女,硕士研究生,研究方向为葡萄与葡萄酒学。E-mail:jiangyuela@nwsuaf.edu.cn
*通信作者简介:惠竹梅(1969—),女,教授,博士,研究方向为葡萄生理生态、葡萄与葡萄酒品质。E-mail:xizhumei@nwsuaf.edu.cn