Screening and Evaluation of Probiotics for Antimicrobial Activity against Foodborne Pathogenic Bacteria
DAI Lu, YUAN Yahong, ZHANG Yuxiang, WEI Jianping, YUE Tianli*
(College of Food Science and Engineering, Northwest A&F University, Yangling 712100, China)
Abstract:Cross streak, radial streak, agar diffusion and co-culture methods were used to assess the antimicrobial activity of various probiotics and their metabolites against four representative foodborne pathogenic bacteria. The probiotic strains with great antibacterial activity against both Gram-positive pathogens (Listeria monocytogenes and Staphylococcus aureus)and Gram-negative pathogens (Escherichia coli and Salmonella typhimurium) were screened out. They were incorporated into polymeric materials (hydroxypropyl cellulose, glycerol and konjac flour) to develop bacteriostatic films, and the probiotic viability and the antimicrobial effects of films were measured. The consistent results obtained from cross streak and radial streak tests showed that Lactobacillus and Enterococcus especially Lactobacillus paracasei CICC 20241 exhibited high antibacterial activity against four target pathogens; the agar diffusion test showed that the lactic acid bacteria culture (LC),cell-free supernatant (CFS), and bacterial cells (BC) of L. paracasei CICC 20241 all had great antibacterial effect, and the co-culture test showed that L. paracasei CICC 20241 exhibited higher growth activity and antagonistic activity when inoculated earlier than the pathogens. In addition, the bacteriostatic film prepared in this experiment was a good carrier for L. paracasei CICC 20241, which was suitable for the survival of the Lactobacillus strain and exhibited continuous antibacterial activity against the target pathogens. The Lactobacillus film may have a great application potential for the development of packaging materials for fresh foods.
Keywords:probiotics; antimicrobial activity; pathogens; bioactive composite film
In spite of the improvement of health management system and the enhancement of safety and hygiene supervision, the outbreak of foodborne diseases caused by pathogenic bacteria contaminating food still occurs around the world[1]. In addition to posing a serious public health threat, the loss of food safety by pathogens often leads to product recalls, causing significant economic losses to the food industry[2]. Because of the high incidence in foodborne illness and the emergence of new virulent serotypes and transmission routes, the contamination of Gram-negative pathogens represented by Escherichia coli and Salmonella spp., is one of the main problems of food safety.As for Gram-positive pathogens, Listeria monocytogenes is considered the most pre-eminent foodborne pathogen because it can survive and grow in various food substrates and environmental conditions[2]. In addition, its wide distribution makes Staphylococcus spp. able to easily contaminate foods,leading to infection or food poisoning incidents. Pathogenic bacteria may cause contamination of fresh products through dust, excreta, irrigation water, or cross-contamination through post-harvest and washing processes.
Physical, chemical or biological strategies used to inhibit or even kill foodborne pathogens are well worth studying. Physical control techniques, such as heat treatment, can improve food safety and shelf life, but also reduce the nutritional and organoleptic properties of the products[3]. As to chemical control, antibiotics are representative antimicrobial agents that are very effective against pathogens and have been commonly used over the past few decades. However, the use of antibiotics has caused many problems and imposed risks. Antibiotics are not specific towards pathogens but broad-spectrum bactericidal,which may result in imbalances in normal flora. The use of antibiotics has caused antimicrobial resistance in pathogenic microorganisms and the residual antibiotics could be ingested by the body[4]. While they have been used routinely in many countries in food animal or produce production operations,antibiotics are disallowed from being used as food additives.Therefore, the development of alternatives or substitutes to antibiotics is very necessary. Microbial antagonism, as a biological control method, utilizes the activity or metabolites of innocuous or beneficial bacterial to control the growth and reproduction of pathogenic bacteria. Since ancient times, living microorganisms have been unintentionally used to inhibit harmful bacteria in fermented foods, giving some enlightenment into the use of microbial antagonism to control pathogens along the food chain to enhance food safety[2,5]. In addition, based on the fact that sanitation measures can not completely eliminate the threat of foodborne pathogens and based on the demand of reducing the use of chemical preservatives and artificial additives,the biocontrol strategy represented by microbial antagonism shows a great advantage[1].
Currently, the most widely accepted antagonistic bacteria are lactic acid bacteria (LAB), identified as ‘qualified presumption of safety’ (QPS) and ‘generally recognized as safe’ (GRAS)[6], which inhibit other microorganisms through different mechanisms, such as competition for nutrients,competition for binding sites, stimulation of immunity, or production of antimicrobial metabolites[7-8]. The antibacterial metabolites produced by LAB, such as lactic acid, diacetyl,acetoin, hydrogen peroxide, bacteriocins and the like, may be used as natural alternatives for chemical preservatives and be potentially applied to food preservation[9-10]. The biological antagonism of LAB has a great potential in the food industry to not only control the growth of pathogens in foods and reduce the incidence of foodborne diseases, but also reduce the growth of spoilage bacteria and prolong the shelf-life of food to improve food safety.
Recently, the application of LAB as protective cultures to control the growth of spoilage and pathogenic bacteria in food has received widespread attention and research[11-13]. Several studies have reported that selected LAB protective cultures can control L. monocytogenes in different food systems[14-15].Antibacterial effects can be achieved by mixing or surface inoculating probiotic protective cultures with food to produce antimicrobial substances during food processing and storage. In sauces, desserts, sausages, meats or dairy products, inoculation of antagonistic strains or addition of bacteriocins leads to inactivation of Staphylococcus aureus[16]. With different antagonists, the growth of E. coli and S. aureus and the production of staphylococcal toxin can be effectively inhibited to varying degrees[11,17-18].
There are several classic methods that can be used to test the antibacterial activity of probiotic isolates. In order to obtain reliable and significant results, cross streak method, radial streak method, agar diffusion method and co-culture method were used to screen out the probiotic strain with the highest antagonistic activity against representative pathogens. The obtained probiotic strain was encapsulated into macromolecular material to prepare a bioactive film. The physical property,security and antimicrobial effect of the film towards foodborne pathogens were evaluated.
1 Materials and Methods
1.1 Raw material and additives
Table 1 Probiotic strains and pathogen strains used in this study
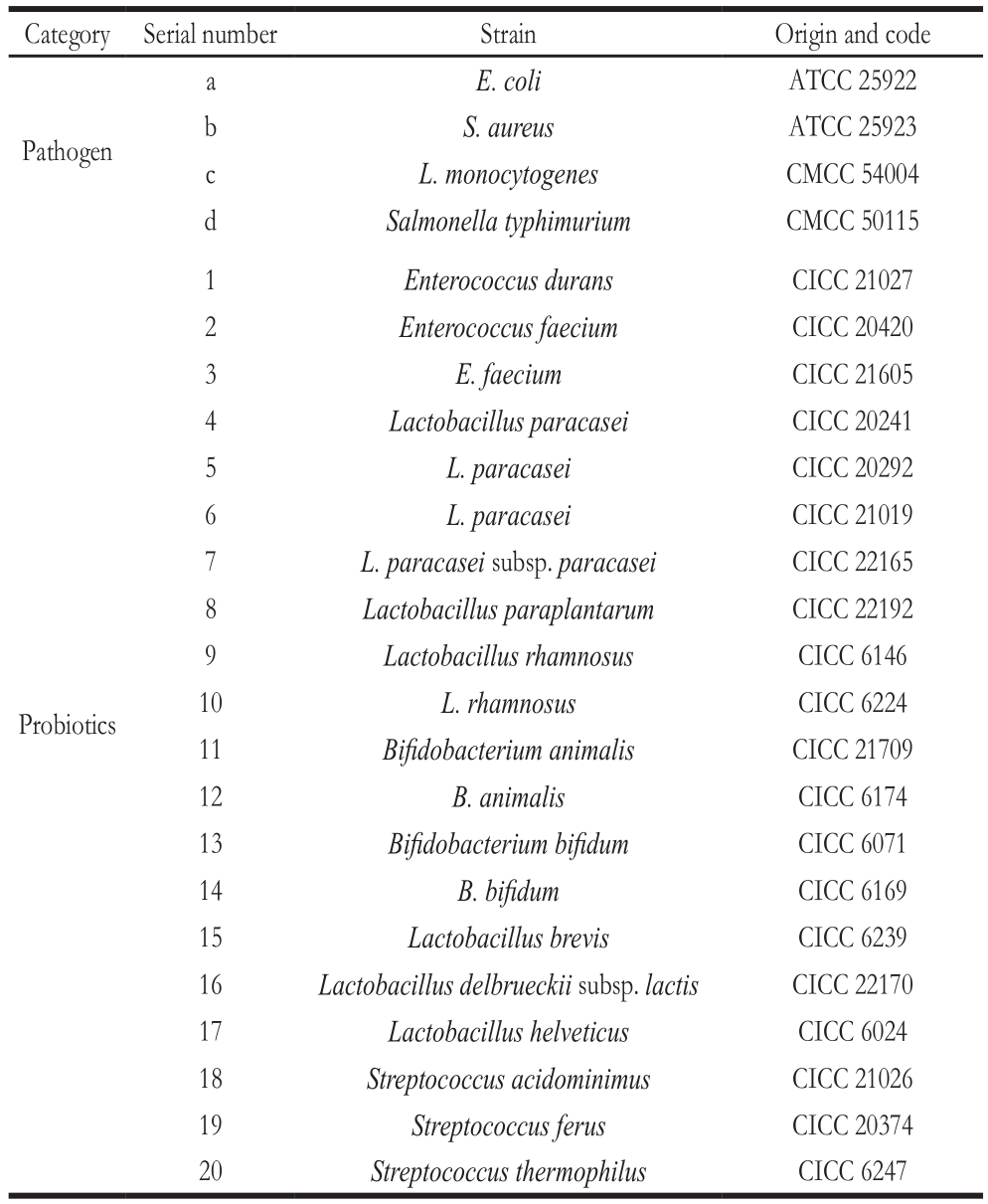
Category Serial number Strain Origin and code E. coli ATCC 25922 b S. aureus ATCC 25923 c L. monocytogenes CMCC 54004 d Salmonella typhimurium CMCC 50115 a Pathogen Enterococcus durans CICC 21027 2 Enterococcus faecium CICC 20420 3 E. faecium CICC 21605 4 Lactobacillus paracasei CICC 20241 5 L. paracasei CICC 20292 6 L. paracasei CICC 21019 7 L. paracasei subsp. paracasei CICC 22165 8 Lactobacillus paraplantarum CICC 22192 9 Lactobacillus rhamnosus CICC 6146 10 L. rhamnosus CICC 6224 11 Bifidobacterium animalis CICC 21709 12 B. animalis CICC 6174 13 Bifidobacterium bifidum CICC 6071 14 B. bifidum CICC 6169 15 Lactobacillus brevis CICC 6239 16 Lactobacillus delbrueckii subsp. lactis CICC 22170 17 Lactobacillus helveticus CICC 6024 18 Streptococcus acidominimus CICC 21026 19 Streptococcus ferus CICC 20374 20 Streptococcus thermophilus CICC 6247 1 Probiotics
The probiotic strains subject of this study were procured from China Center of Industrial Culture Collection (CICC,Beijing, China) and standard pathogenic strains were purchased from American Type Culture Collection (ATCC,Manassas, VA, USA) and National Center for Medical Culture Collections (CMCC, Beijing, China)(Table 1).Bacterial strains were respectively incubated at 37 ℃ for 24 h followed by two successive transfers to their broth, MRS broth (AOBOX, Beijing, China) for lactobacilli and TSB broth (AOBOX, Beijing, China) for pathogens.
Hydroxypropyl cellulose (HPC, CSA 9004-64-2)and konjac flour (KF, commercial grade with 76.3% KGM)were respectively obtained from Shanghai Yuanye Biological Technology Co. Ltd. and Shaanxi Jintai Konjac Industry Development Co. Ltd.. Glycerol (GL, CSA 56-81-5) purchased from Sichuan Xilong Chemical Co. Ltd. was used as a plasticizer. Cell Counting Kit-8 (CCK8) was acquired from MCE Medchemexpress Co. Ltd..
1.2 Instrument and apparatus
Electric heating incubator (DPX-9002B-1) was produced by Shanghai Foma Experimental Equipment Co. Ltd.; High speed refrigerated centrifuge (HC-3018R) was produced by Anhui Zhongke Scientific Instrument Co. Ltd.; Vortex shaker(wi1102) was produced by Dongyi Technology Co. Ltd.;Ultra-clean worktable (BCN-1360B) was produced by Harbin Donglian Electronic Technology Development Co. Ltd.;High pressure steam sterilization pot (YXQ-LS-50A) was produced by Shanghai Boson Industrial Co. Ltd.; Texture analyzer (TA.XT Plus) was produced by the United Kingdom Stable Micro Systems Co. Ltd.; Multi-angle gloss meter(HP-386) was produced by Shanghai Puxi Optoelectronics Technology Co. Ltd..
1.3 Methods
1.3.1 Cross streak method
Antimicrobial activity of the probiotic strains against pathogenic strains was measured using a modified ‘crossstreak technique’[19]. MRS agar plates were streaked with the probiotic strains (108CFU/mL) in the center of the plate covering a 1 cm × 2 cm area and incubated in anaerobic conditions at 37 ℃ for 48 h. When probiotic growth became confluent, the probiotic colonies were removed and the residual cells were inactivated by immersing into chloroform for 1 h. After aeration for 1 h, the pathogen(107–108CFU/mL) was streaked onto the plates and incubated at 37 ℃ for 24 h. The inhibition activity of the probiotic strains was evaluated by measuring the zone of inhibition around probiotics growth.
1.3.2 Radial streak method
Microbial interactions between probiotic strains and pathogenic strains were investigated by a radial streak method. MRS agar plates were inoculated with the probiotic inoculum (108CFU/mL) in the center of the plate covering a circle area with diameter of 2.5 cm and incubated anaerobically at 37 ℃ for 48 h[19]. After then, the plates were streaked by radial lines of pathogen strains suspensions(107–108CFU/mL) from border to center. After 24 h of incubation at 37 ℃, the growth inhibition (GI) was calculated by subtracting probiotics circle diameter from inhibition zone diameter and then dividing by two.
1.3.3 Agar diffusion method
The antibacterial effect of probiotic strains was analyzed by an agar diffusion method. Each of probiotic strains was cultivated in MRS broth for 24 h at 37 ℃ to acquire the LAB culture (LC). The cell-free supernatant (CFS) was obtained by centrifuging the culture at 10 000 × g for 10 min and sterilized by filtration using 0.22 μm porous membranes. The remaining bacterial cells (BC) after centrifugation were resuspended to prepare suspensions. In order to determine the inhibitory activities of LC, CFS and BC of each probiotics,agar well diffusion method[20]was adopted. 10 mL of TSB(1.5% agar) medium was poured into a sterilized petri dish as the lower layer. After solidification, the sterilized oxford cups with diameter of 8 mm were put onto the TSB agar.20 mL of TSB (1.5% agar) medium inoculated with an overnight culture of the pathogen strains in the stationary phase (107–108CFU/mL) was then poured onto the TSB plates as the upper medium. After removing oxford cups,100 μL of each LC, CFS, and BC was added into each well.The plates were incubated at 37 ℃ for 24 h, and the diameters of inhibition zone were recorded as the diameters of growthfree inhibition zones minus oxford cup diameter.
1.3.4 Co-culture method
The interference of LAB with the growth of pathogens was evaluated by a co-culture method[21]. The co-culture tests were conducted in a mixed medium, MRS broth and TSB broth 1:1, capable of sustaining the growth of both probiotics and pathogens. The co-culture experiments were divided into three groups: Group A, 1 mL of LAB suspension (108CFU/mL)and 1 mL of each pathogenic suspension (107–108CFU/mL)were simultaneously inoculated into 100 mL of mixed medium and incubated at 37 ℃ for 24 h. Group B, LAB suspension (108CFU/mL) was inoculated and incubated for 8 h before the addition of pathogenic suspension (107–108CFU/mL). Group C, pathogenic suspension (107–108CFU/mL) was inoculated prior to the addition of LAB suspension (108CFU/mL). After co-culture, the gradient diluted co-culture suspensions were plated on MRS or TSB agar medium and incubated at 37 ℃ for 24–48 h for counting. The LAB strain or the pathogen strains was inoculated to the same medium for positive controls,respectively. The antagonism effects were calculated by dividing co-culture counts by control counts and then multiplying by one hundred.
1.3.5 Preparation and physical property of bioactive composite films
According to pre-experiment, 0.8% (m/m) of HPC,1.5 mL/100 g of GL and 1.0% (m/m) of KF were successively dissolved in distilled water under continuous stirring for dispersion. After adequate hydration and interactions, 1% L. paracasei CICC 20241 suspensions were incorporated into the film-forming dispersions with a final concentration of 108CFU/mL, and the dispersions were stirred for homogenization and degassed under a vacuum. Bioactive films were obtained by pouring 5 mL of dispersions onto a polystyrene plate (φ = 6 cm) and drying at 65% relative humidity (RH) and 25 ℃ for approximately 48 h. Films without bacterial cells were made and taken as controls. Mechanical property is awfully important for packaging materials to maintain integrity during processing,transportation, and storage[22]. The tensile strength (TS),elongation percentage at break (EAB), water vapor permeability (WVP), light transmittance (LT) and haze(H) of LAB bioactive composite films were determined in accordance with GB/T 1040—2006 Plastics determination of tensile properties, GB/T 1037—1988 Test method for water vapor transmission of plastic film and sheet cup method,and GB/T 2410—2008 Determination of the luminous transmittance and haze of transparent plastics, respectively.1.3.6 Safety evaluation and antimicrobial activity of bioactive composite films
To ensure the safety of LAB bioactive composite films, CCK8 method was used to assess its cytotoxicity,and HepG2 and BGC-823 were selected as cell models for in vitro toxicity studies[23-24]. 100 μL of diluted HepG2 and BGC-823 (104cells/well) cell suspensions were plated in a 96-well plate, and various concentrations of LAB bioactive composite films were added to each well (final concentration 0, 0.2, 2, 20 μg/mL and 200 μg/mL) were cultured at 37 ℃, 95% RH, and 5%CO2for 6, 12 h, and 24 h, respectively. Thereafter, 10 μL of CCK solution (10%, m/m) was added to each well, and then the absorbance at 450 nm was measured after reaction for 1 h. To evaluate the survivability of lactobacilli in films and antimicrobial activity of the films, the TSB (1.5% agar)medium plates were inoculated with 100 μL of pathogenic suspension (107–108CFU/mL) and the composite films were placed on the surfaces of the plates. The plates were placed at 65% RH and 5 ℃ to simulate the usual conditions used to preserve fresh foodstuffs or agricultural products. The pathogens and L. paracasei CICC 20241 counts on plates were periodically performed during the storage time.
1.4 Data analysis
All measurements were performed in triplicate. The data were analyzed by analysis of variance (ANOVA) using SPSS software (SPSS Inc., version 13.0). Duncan’s multiple range tests were used to estimate significant differences among mean values. Values are given as ± s deviation and significance was defined at a level of P < 0.05.
2 Results and Analysis
2.1 Antimicrobial activity by cross streak method
Table 2 Inhibition of pathogenic strains by probiotic strains

Note: –. no inhibition; +. inhibition zone < 1 cm × 2 cm; ++. inhibition zone <1.5 cm × 2.5 cm; +++. inhibition zone < 2 cm × 3 cm; ++++. inhibition zone >2 cm × 3 cm.
S. typhimurium CMCC 50115 B. animalis CICC 21709 –+++++–B. animalis CICC 6174 –++++–B. bifidum CICC 6071 –++–B. bifidum CICC 6169 –+++–E. durans CICC 21027 + ++ +++ ++E. faecium CICC 20420 ++ +++ +++ +E. faecium CICC 21605 ++ +++ ++ +L. brevis CICC 6239 –+++–L. delbrueckii subsp. lactis CICC 22170 + ++ ++ –L. helveticus CICC 6024 –+++++–L. paracasei CICC 20241 +++ ++++ +++ ++L. paracasei CICC 20292 + +++ +++ +L. paracasei CICC 21019 ++ +++ +++ +L. paracasei subsp. paracasei CICC 22165 + +++ +++ ++L. paraplantarum CICC 22192 ++ +++ +++ ++L. rhamnosus CICC 6146 + +++ +++ ++L. rhamnosus CICC 6224 ++ +++ ++ ++S. acidominimus CICC 21026–++++++–S. ferus CICC 20374 –++++++–S. thermophilus CICC 6247–++++–Probiotic strains Inhibition of growth of pathogens E. coli ATCC 25922 S. aureus ATCC 25923 L. monocytogenes CMCC 54004
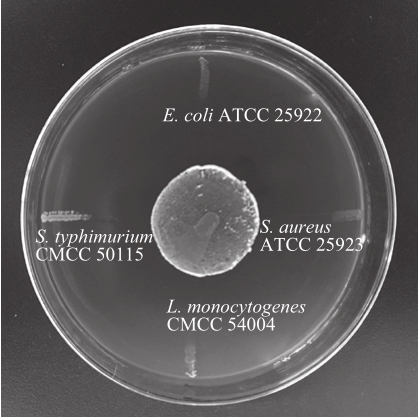
In cross streak experiment, as seen in Table 2, almost all of probiotic strains involved in the experiment had differing degrees of inhibition on S. aureus ATCC 25923 and L. monocytogenes CMCC 54004, both of which are Gram-positive bacteria. Among the antibacterial probiotics,L. paracasei, L. paraplantarum, L. rhamnosus, S.acidominimus, S. ferus and E. faecium strains exhibited relatively higher antibacterial activity against Grampositive bacteria. However, quite a few probiotic strains presented no bacteriostatic effect on E. coli ATCC 25922 or S. typhimurium CMCC 50115, such as B. animalis, B. bifidum,L. helveticus, and S. thermophilus. Fig. 1 shows the inhibitory effect of L. paracasei CICC 20241 against pathogenic bacteria.Actually, probiotic strains are much more effective against Gram-positive bacteria than Gram-negative bacteria[25]. In general, the results showed that Lactobacillus and Enterococcus strains, especially L. paracasei strains, have higher antibacterial activity against all pathogens in the experiment, both Grampositive and Gram-negative bacteria. For the sake of verification the experimental results, radial streak method was conducted to achieve mutual agreement.
2.2 Antimicrobial activity by radial streak method
Fig. 2 showed the GI values of probiotic strains against pathogen strains, in which the highest GI value ((17.8 ± 0.45)mm) was detected by L. paracasei CICC 20241 against S.aureus ATCC 25923, whereas the lowest GI value ((1.2 ±0.24) mm) was observed by S. ferus CICC 20374 against S. typhimurium CMCC 50115. The results of radial streak experiment have the same bacteriostasis tendency as the results in cross streak experiment (Table 2). Most probiotic strains can effectively inhibit the growth of Gram-positive bacteria, while most of the probiotic strains were not efficient in inhibition of Gram-negative bacteria. Fig. 3 shows the inhibitory effect of L. paracasei CICC 20241 against pathogenic bacteria. Based on the results of radial streak and cross streak experiments, 10 strains of L. paracasei, L. paraplantarum, L. rhamnosus, and E. durans, which can significantly inhibit both Gram-positive and Gram-negative pathogens, were selected to further examine the bacteriostatic effect and screen for the best bacteriostatic strains.
2.3 Antimicrobial activity by agar diffusion method
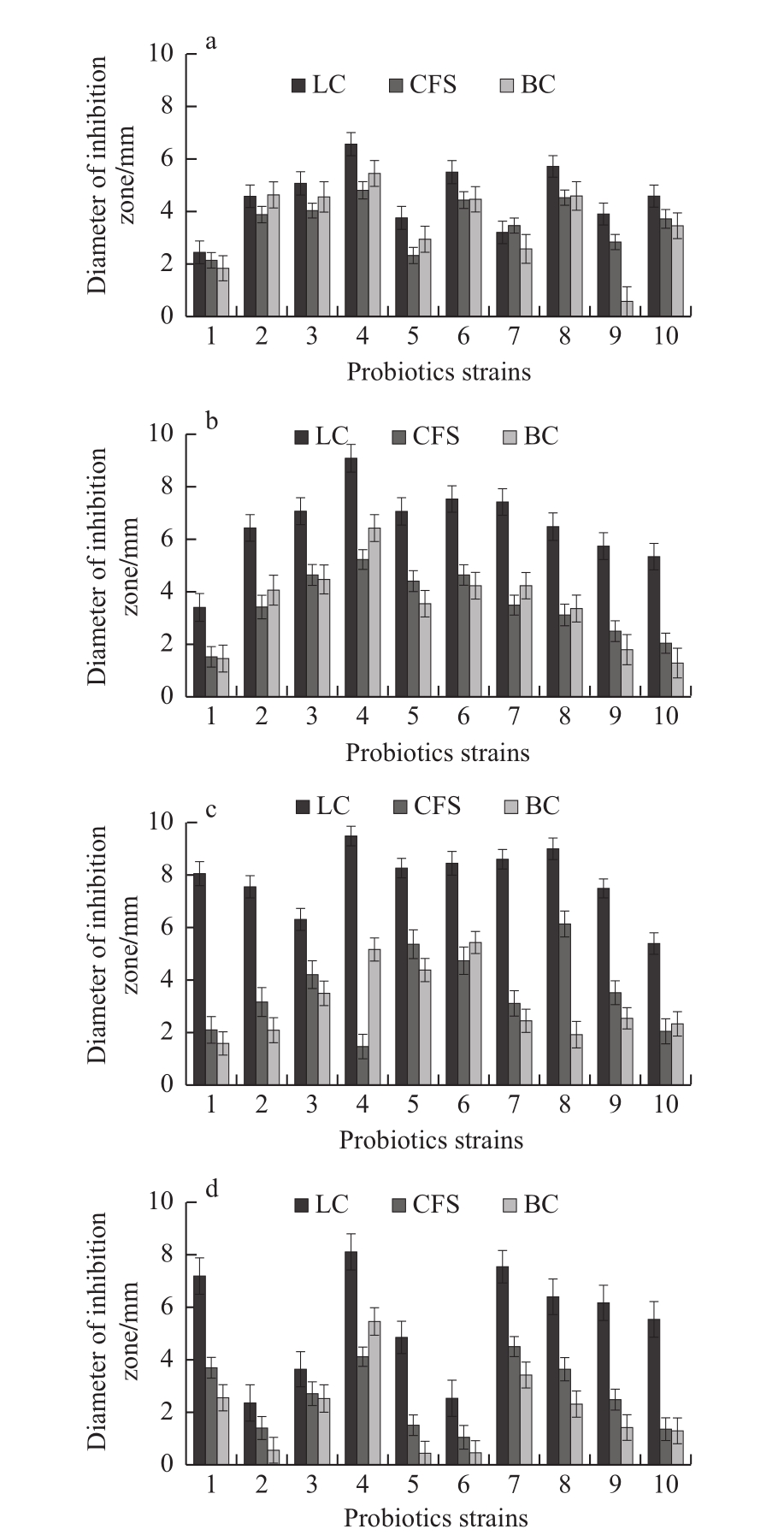
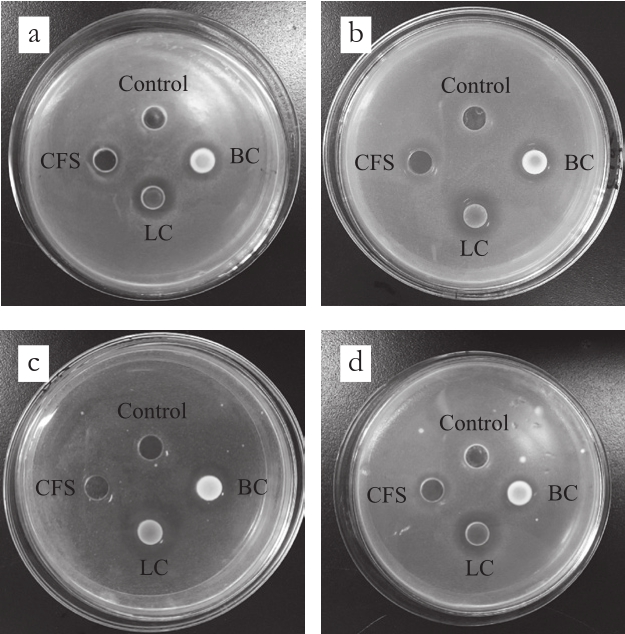
The antibacterial effects of LC, CFS, and BC of each probiotic strain on pathogens by diffusion method were shown in Fig. 4. The inhibition zones produced by the diffusion method (Fig. 5) were relatively smaller than the ones by the streak method, which were consistent with the reported researches[19,26]. Due to competition for nutrients or production of bacteriocins, hydrogen peroxide, organic acids and ethyl alcohol, a great many probiotic strains presented antibacterial ability against spoilage and pathogenic microorganisms[27-28].The bacteriostatic effects of CFS were better than that of BC,while for some other strains, the bacteriostatic effects of BC were better than CFS. In any case, the antibacterial effects of LC were the best for almost all probiotic strains. The results of this experiment may be ascribed to the different metabolic characteristics of each probiotic strain, the production of different kinds or quantity of antibacterial metabolites and the accumulation and diffusion of these metabolites[19]. The results of diffusion experiment were consistent with the results of cross and radial streak experiments, L. paracasei CICC 20241 was confirmed as the strain with highest antibacterial activity.
2.4 Antimicrobial activity by co-culture method
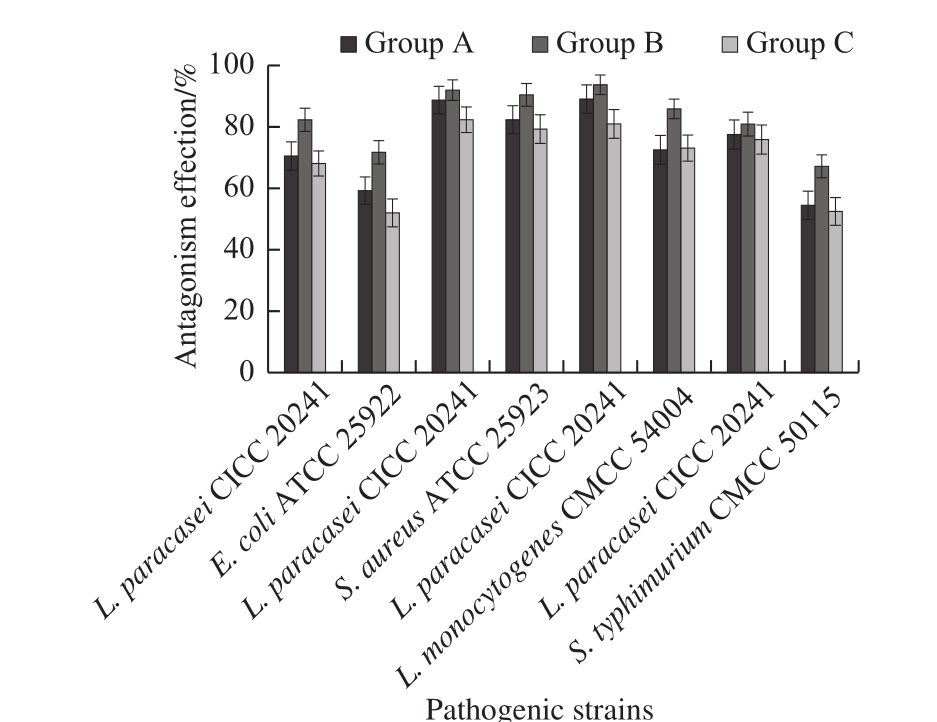
The inhibitory effect of lactobacillus on the growth of pathogens in liquid medium was evaluated by observing the growth of the co-cultures after cultivation for 24 h on agar plates. The results of the co-culture of L. paracasei CICC 20241 with pathogenic bacteria in MRS/TSB mixed media were shown in Fig. 6. In three sets of experiments, the growth rates of four pathogens were inhibited to varying degrees by lactobacillus as compared to the control, and the L. paracasei CICC 20241 was highly antagonistic to all pathogens, with the antagonism effects against Gram-positive bacteria higher than that against Gram-negative bacteria. In each Group B experiment, lactobacillus inoculated before the addition of pathogenic bacteria, the lactobacillus showed higher growth activity and antagonistic activity when compared to Group A or C. The antagonistic activity in liquid co-culture experiment could be attributed to rapid diffusion of antimicrobial metabolites from lactobacillus strains and co-aggregation of lactobacillus strains with pathogenic bacteria[19,29-30].
2.5 Physical property and safety evaluation of bioactive composite films
TS of (57.44 ± 8.30) MPa and EAB of (26.50 ± 4.25)%are consistent with the requirement of GB 10457—2009,indicating the LAB bioactive composite films with enough mechanical strength. WVP is a crucial indicator to measure the ability of packaging materials to prevent moisture transfer between food and environment. The WVP of LAB bioactive composite films was (0.82 ± 0.11) ×1010g/(m·s·Pa), which is in line with GB standard. The optical property reflects the practicability and acceptability of packaging materials.LT and haze (H) of LAB bioactive composite films were(96.67 ± 2.43)% and (2.85 ± 0.60)%, which were conform to the standard provisions.
Table 3 Cytotoxicity evaluation of bioactive composite films
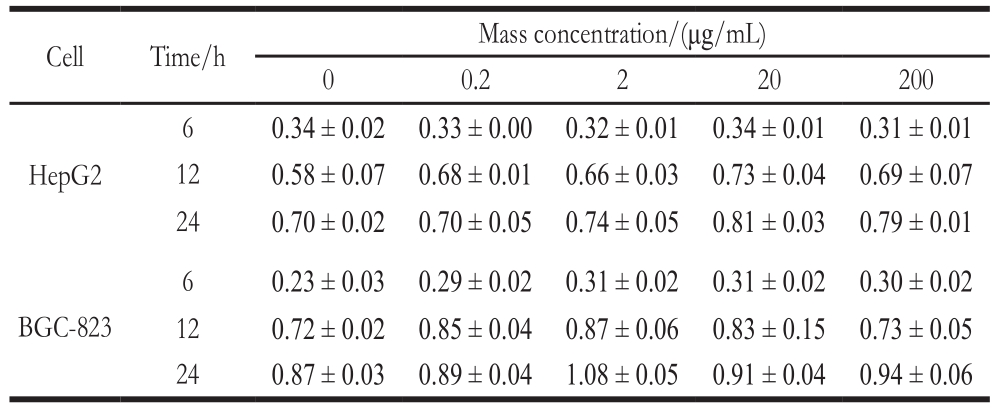
Cell Time/h Mass concentration/(μg/mL)0 0.2 2 20 200 HepG2 6 0.34 ± 0.02 0.33 ± 0.00 0.32 ± 0.01 0.34 ± 0.01 0.31 ± 0.01 12 0.58 ± 0.07 0.68 ± 0.01 0.66 ± 0.03 0.73 ± 0.04 0.69 ± 0.07 24 0.70 ± 0.02 0.70 ± 0.05 0.74 ± 0.05 0.81 ± 0.03 0.79 ± 0.01 BGC-823 6 0.23 ± 0.03 0.29 ± 0.02 0.31 ± 0.02 0.31 ± 0.02 0.30 ± 0.02 12 0.72 ± 0.02 0.85 ± 0.04 0.87 ± 0.06 0.83 ± 0.15 0.73 ± 0.05 24 0.87 ± 0.03 0.89 ± 0.04 1.08 ± 0.05 0.91 ± 0.04 0.94 ± 0.06
As the results of cytotoxicity assessment shown in Table 3, when the treatment time was constant while the concentration of the films increases, all the HepG2 and BGC-823 cells showed almost no significant difference(P > 0.05). When the concentration of the films was constant,the growth of HepG2 and BGC-823 cells was not inhibited when the treatment time was extended. These indicate that long-time treatment of BGC-823 and BGC-823 cells by highconcentration films had no significant effect on cell activity,thus the LAB bioactive composite films was considered not to be cytotoxic.
2.6 Antimicrobial activity of bioactive composite films
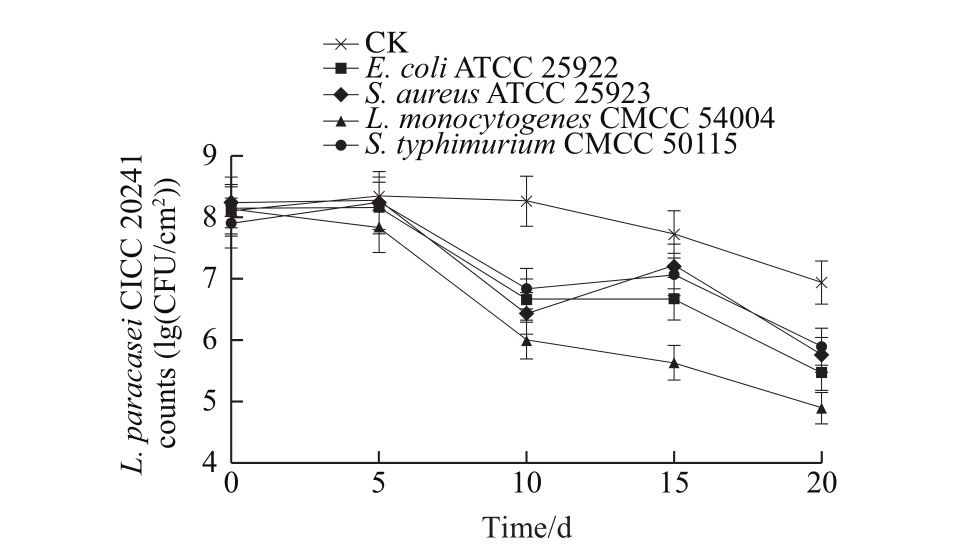
The survival of L. paracasei CICC 20241 in the films during storage can be seen in Fig. 7. The L. paracasei CICC 20241 included in the film had a growth phase at the primary stage, followed by a relatively stable phase, and subsequently a mild descent until the end of the storage time. The composite films, appear to be an appropriate environment for L. paracasei CICC 20241 to live in, and thus its population was maintained at more than 86% of the initial amount to the end of storage period. When the films contact with E. coli, S. aureus and S. typhimurium, the L. paracasei CICC 20241 counts were slightly increased at the initial stage and then gradually decreased by 2.66, 2.47 and 2.00 (lg(CFU/cm2)), respectively. As for the experiment with L. monocytogenes, the L. paracasei CICC 20241 counts gradually decreased up to 4.91 (lg(CFU/cm2)) at the end of the experiment. The decreases in the L. paracasei CICC 20241 counts are likely a consequence of interactions,antagonism, or competition between lactobacillus and target bacteria.
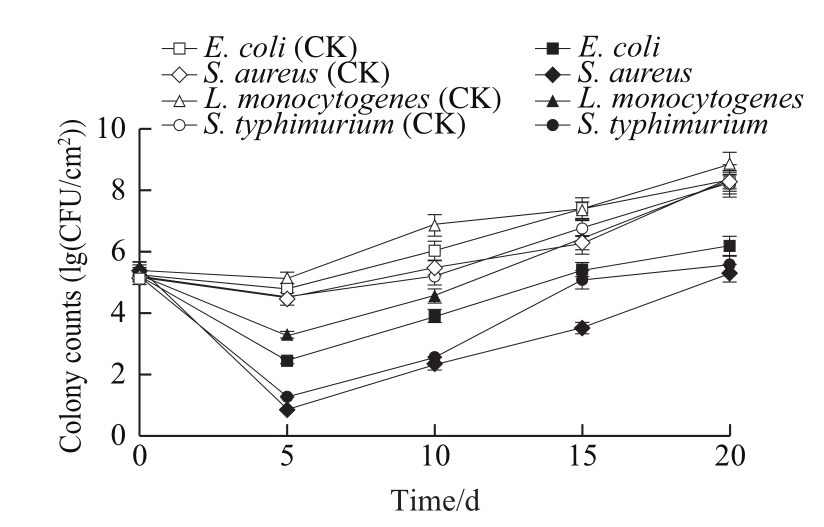
The antimicrobial effects of the films containing L.paracasei CICC 20241 were determined and are shown in Fig. 8. The pathogenic counts increased to 8 (lg(CFU/cm2))at the end of the experiment in control plates. The films containing L. paracasei CICC 20241 presented superior bactericidal activities against S. aureus, S. typhimurium and E. coli. Following an initial 5 d, a sharp reduction in microbial population of almost 3 (lg(CFU/cm2)) compared to the control was observed. Afterwards, the films still remained efficient in inhibiting microbial growth during the whole storage period. However, the films including L. paracasei CICC 20241 exhibited a weaker antibacterial effect against L.monocytogenes, led to a relatively low reduction of microbial growth. The pathogen strains have their own suitable growth conditions and have different adaptabilities towards different environmental conditions, among which L. monocytogenes has exceptional adaptation to low temperature conditions[21].In addition, the antimicrobial activity of lactobacillus depends on many factors such as environmental growth conditions,which could affect the metabolic characteristics (the production of different kinds and quantities of metabolites)[19].Therefore, this may be why the antibacterial effect under simulated refrigerated conditions is different from the previous experimental results. Presently, the components ratio of the composite films might not be necessarily the most suitable for the growth and metabolism of lactobacillus, thus its optimization could be a method to improve the adaptability and antibacterial effect of lactobacillus in the films, which will be further studied.
The antimicrobial experimental results show that the incorporation of L. paracasei CICC 20241 into the composite films was a feasible approach at controlling the growth of representative pathogens. The antibacterial potential of the bioactive films against pathogenic organisms could be essentially attributed to the synergistic effect of antimicrobial metabolites resulting from the antagonistic or competitive effect of L.paracasei CICC 20241 against pathogens[31-32].
3 Conclusion
In this work, four kinds of methods were used to screen out the L. paracasei CICC 20241 strain with high antibacterial activity against pathogens, and the antibacterial properties of the L. paracasei CICC 20241 strain were evaluated. The antagonistic strain was incorporated into polymeric materials to prepare bioactive film. The packaging performance and safety assessment indicate that the developed films have great potential as a bioactive packaging for fresh food. The survival of lactobacillus in the film and the antibacterial effect of the film were also discussed. The film proved to be suitable for the survival of L. paracasei CICC 20241 and exhibited sustained antibacterial activity towards representative food pathogens.This study was limited in scope, we intend to further this work by focusing not only on the antimicrobial properties of the lactobacillus, but also on the isolation and identification of the metabolites, their mechanisms of action, and potential applications.
References:
[1] JORDAN K, DALMASSO M, ZENTEK J, et al. Microbes versus microbes: control of pathogens in the food chain[J]. Journal of the Science of Food & Agriculture, 2014, 94(15): 3079-3089.DOI:10.1002/jsfa.6735.
[2] GLVEZ A, ABRIOUEL H, BENOMAR N, et al. Microbial antagonists to food-borne pathogens and biocontrol[J]. Current Opinion in Biotechnology, 2010, 21(2): 142-148. DOI:10.1016/j.copbio.2010.01.005.
[3] DI C R, SURICO R F, SIRAGUSA S, et al. Selection and use of autochthonous mixed starter for lactic acid fermentation of carrots, French beans or marrows[J]. International Journal of Food Microbiology, 2008, 127(3): 220-228. DOI:10.1016/j.ijfoodmicro.2008.07.010.
[4] BUDI O F E L, THOMAZ M C, KRONKA R N, et al. Effect of probiotic and prebiotic inclusion in weaned piglet diets on structure and ultra-structure of small intestine[J]. Brazilian Archives of Biology & Technology, 2005, 48(6): 921-929. DOI:10.1590/s1516-89132005000800008.
[5] RESTUCCIA C, GIUSINO F, LICCIARDELLO F, et al. Biological control of peach fungal pathogens by commercial products and indigenous yeasts[J]. Journal of Food Protection, 2006, 69(10): 2465-2470. DOI:10.4315/0362-028x-69.10.2465.
[6] GLVEZ A, ABRIOUEL H, LPEZ R L, et al. Bacteriocin-based strategies for food biopreservation[J]. International Journal of Food Microbiology, 2007, 120(1/2): 51-70. DOI:10.1016/j.ijfoodmicro.2007.06.001.
[7] DU J F, MIAO L H, MA H H, et al. Screening and identification of lactic acid bacteria antagonistic against Enterobacter sakazakii[J]. Food Science,37(11): 125-130. DOI:10.7506/spkx1002-6630-201611022.
[8] ZHANG X M, ZHAO Y X, YAN X J, et al. Screening and identification of lactic acid bacterium with antimicrobial and antioxidant activity[J].Food Science, 2018, 39(2): 93-98. DOI:10.7506/spkx1002-6630-201802015.
[9] HARP E, GILLILAND S E. Evaluation of a select strain of Lactobacillus delbrueckii subsp. lactis as a biological control agent for pathogens on fresh-cut vegetables stored at 7 degrees C[J]. Journal of Food Protection,2003, 66(6): 1013-1018. DOI:10.4315/0362-028x-66.6.1013.
[10] LI Y, WEI X Y, WANG J, et al. Isolation and identification of lactic acid bacteria inhibiting Penicillium and analysis of their antimicrobial components[J]. Food Science, 2015, 36(21): 150-155. DOI:10.7506/spkx1002-6630-201521029.
[11] REVOLLEDO L, FERREIRA C S, FERREIRA A J. Prevention of Salmonella typhimurium colonization and organ invasion by combination treatment in broiler chicks[J]. Poultry Science, 2009,88(4): 734-743. DOI:10.3382/ps.2008-00410.
[12] LUCIANO F B, BELLAND J, HOLLEY R A. Microbial and chemical origins of the bactericidal activity of thermally treated yellow mustard powder toward Escherichia coli O157:H7 during dry sausage ripening[J]. International Journal of Food Microbiology, 2011, 145(1):69-76. DOI:10.1016/j.ijfoodmicro.2010.11.028.
[13] SENNE M M, GILLILAND S E. Antagonistic action of cells of Lactobacillus delbrueckii subsp. lactis against pathogenic and spoilage microorganisms in fresh meat systems[J]. Journal of Food Protection,2003, 66(3): 418-425. DOI:10.4315/0362-028x-66.3.418.
[14] TOM E, GIBBS P A, TEIXEIRA P C. Growth control of Listeria innocua 2030c on vacuum-packaged cold-smoked salmon by lactic acid bacteria[J]. International Journal of Food Microbiology, 2008,121(3): 285-294. DOI:10.1016/j.ijfoodmicro.2007.11.015.
[15] MARAGKOUDAKIS P A, MOUNTZOURIS K C, PSYRRAS D, et al.Functional properties of novel protective lactic acid bacteria and application in raw chicken meat against Listeria monocytogenes and Salmonella enteritidis[J]. International Journal of Food Microbiology,2009, 130(3): 219-226. DOI:10.1016/j.ijfoodmicro.2009.01.027.
[16] JOFR A, GARRIGA M, AYMERICH T. Inhibition of Salmonella sp. Listeria monocytogenes and Staphylococcus aureus in cooked ham by combining antimicrobials, high hydrostatic pressure and refrigeration[J]. Meat Science, 2008, 78(1/2): 53-59. DOI:10.1016/j.meatsci.2007.06.015.
[17] UKUKU D O, ZHANG H, HUANG L. Growth parameters of Escherichia coli O157:H7, Salmonella spp., Listeria monocytogenes,and aerobic mesophilic bacteria of apple cider amended with nisin-EDTA[J]. Foodborne Pathogens & Disease, 2009, 6(4): 487-494.DOI:10.1089/fpd.2008.0233.
[18] SPARO M, NUEZ G G, CASTRO M, et al. Characteristics of an environmental strain, Enterococcus faecalis CECT7121, and its effects as additive on craft dry-fermented sausages[J]. Food Microbiology,2008, 25(4): 607-615. DOI:10.1016/j.fm.2008.01.008.
[19] COMAN M M, VERDENELLI M C, CECCHINI C, et al. In vitro evaluation of antimicrobial activity of Lactobacillus rhamnosus IMC 501®, Lactobacillus paracasei IMC 502® and SYNBIO® against pathogens[J]. Journal of Applied Microbiology, 2014, 117(2): 518-527.DOI:10.1111/jam.12544.
[20] ANAS M, EDDINE H J, MEBROUK K. Antimicrobial activity of Lactobacillus species isolated from Algerian raw goat’s milk against Staphylococcus aureus[J]. World Journal of Dairy & Food Sciences,2008, 3(2): 39-49.
[21] MARIAM S H, ZEGEYE N, TARIKU T, et al. Potential of cellfree supernatants from cultures of selected lactic acid bacteria and yeast obtained from local fermented foods as inhibitors of Listeria monocytogenes, Salmonella spp. and Staphylococcus aureus[J]. BMC Research Notes, 2014, 7(1): 1-9. DOI:10.1186/1756-0500-7-606.
[22] SHI Y J, ZHANG H J, ZHANG Z H, et al. The effect of components of soy protein-based gelatin composite films on the mechanical properties[J].Journal of Chinese Institute of Food Science and Technology, 2017, 17(4):123-131. DOI:10.16429/j.1009-7848.2017.04.016.
[23] MA F S, YU Y, ZHANG J, et al. A state-of-the-art review of cytotoxicity evaluation of biomaterials[J]. Materials Review, 2018, 32(1): 76-85.DOI:10.11896/j.issn.1005-023X.2018.01.009.
[24] YU S P, SU X D, DU J L, et al. The cytotoxicity of water-soluble carbon nanotubes on human embryonic kidney and liver cancer cells[J].New Carbon Materials, 2018, 33(1): 36-46. DOI:10.1016/s1872-5805(18)60325-7.
[25] RAVAEI A, POOR Z H, SALEHI T Z, et al. Evaluation of antimicrobial activity of three Lactobacillus spp. against antibiotic resistance Salmonella typhimurium[J]. Advanced Studies in Biology,2013, 7(1): 1-9. DOI:10.12988/asb.2013.13006.
[26] CADIRCI B H, CITAK S. Antagonistic effects of some lactobacilli on some Gram-negative bacteria[J]. Gazi University Journal of Science,2010, 23(2): 43-63.
[27] CALO-MATA P, ARLINDO S, BOEHME K, et al. Current applications and future trends of lactic acid bacteria and their bacteriocins for the biopreservation of aquatic food products[J]. Food &Bioprocess Technology, 2008, 1(1): 43-63. DOI:10.1007/s11947-007-0021-2.
[28] ASURMENDI P, GARC A M J, PASCUAL L, et al. Biocontrol of Listeria monocytogenes by lactic acid bacteria isolated from brewer’s grains used as feedstuff in Argentina[J]. Journal of Stored Products Research, 2015, 61(1): 27-31. DOI:10.1016/j.jspr.2015.02.001.
[29] SHAH N, PATEL A, AMBALAM P, et al. Determination of an antimicrobial activity of Weissella confusa, Lactobacillus fermentum,and Lactobacillus plantarum against clinical pathogenic strains of Escherichia coli and Staphylococcus aureus in co-culture[J]. Annals of Microbiology, 2016, 66(3): 1137-1143. DOI:10.1007/s13213-016-1201-y.
[30] LAVERMICOCCA P, VALERIO F, LONIGRO S L, et al. Antagonistic activity of potential probiotic lactobacilli against the ureolytic pathogen yersinia enterocolitica[J]. Current Microbiology, 2008, 56(2):175-181. DOI:10.1007/s00284-007-9069-5.
[31] GÁLVEZ A, ABRIOUEL H, LÓPEZ R L, et al. Bacteriocinbased strategies for food biopreservation[J]. International Journal of Food Microbiology, 2007, 120(1/2): 51-70. DOI:10.1016/j.ijfoodmicro.2007.06.001.
[32] LONARD L, DEGRAEVE P, GHARSALLAOUI A, et al. Design of biopolymeric matrices entrapping bioprotective lactic acid bacteria to control Listeria monocytogenes growth: comparison of alginate and alginate-caseinate matrices entrapping Lactococcus lactis subsp.lactis cells[J]. Food Control, 2014, 37(1): 200-209. DOI:10.1016/j.foodcont.2013.09.041.
收稿日期:2018-04-12
基金项目:国家自然科学基金面上项目(31371814;31671866);国家重点基础研究发展计划(973计划)项目(2013FY113400)
第一作者简介:戴璐(1988—),女,博士研究生,研究方向为食品科学。E-mail:dailuxn@126.com
*通信作者简介:岳田利(1965—),男,教授,博士,研究方向为食品生物技术与食品安全。E-mail:yuetl305@nwafu.edu.cn
抗食源性致病菌益生菌的筛选及其抑菌活性评价
戴 璐,袁亚宏,张宇翔,魏建平,岳田利*
(西北农林科技大学食品科学与工程学院,陕西 杨凌 712100)
摘 要:采用交叉划线法、径向划线法、琼脂扩散法及液体共培养法评价各益生菌菌株及其代谢产物对4 株具代表性食源性致病菌的抑菌作用,并从20 株益生菌中筛选出同时对单核细胞性李斯特菌和金黄色葡萄球菌(革兰氏阳性致病菌)及大肠杆菌和鼠伤寒沙门菌(革兰氏阴性致病菌)具有良好抑菌活性的益生菌菌株。将筛选出的益生菌添加到羟丙基纤维素、丙三醇及魔芋粉的高分子复合材料中制备生物活性抑菌膜,并对抑菌膜中益生菌的存活情况及抑菌膜的抗菌效果进行评价。交叉划线法及径向划线法实验结果一致:乳杆菌属和肠球菌属的菌株,特别是Lactobacillus paracasei CICC 20241菌株对4 株目标致病菌均具有较高的抗菌活性;琼脂扩散法实验表明:L. paracasei CICC 20241菌株的培养液、上清液和菌悬液均具有较好的抑菌效果;液体共培养法实验表明:乳酸菌L. paracasei CICC 20241先于致病菌接种时表现出更高的生长活性和拮抗活性。此外,本实验制备的抑菌膜是L. paracasei CICC 20241菌株的良好载体,适宜该菌株的生长存活并对目标致病菌表现出持续的抗菌活性,对于开发鲜食食品的包装材料具有较大的应用潜力。
关键词:益生菌;抑菌活性;致病菌;生物活性复合膜
中图分类号:TS201.3
文献标志码:A
文章编号:1002-6630(2018)24-0093-09
引文格式:
DAI Lu, YUAN Yahong, ZHANG Yuxiang, et al. Screening and evaluation of probiotics for antimicrobial activity against foodborne pathogenic bacteria[J]. Food Science, 2018, 39(24): 93-101. DOI:10.7506/spkx1002-6630-201824015. http://www.spkx.net.cn
DAI Lu, YUAN Yahong, ZHANG Yuxiang, et al. Screening and evaluation of probiotics for antimicrobial activity against foodborne pathogenic bacteria[J]. 食品科学, 2018, 39(24): 93-101. DOI:10.7506/spkx1002-6630-201824015. http://www.spkx.net.cn
DOI:10.7506/spkx1002-6630-201824015