活性羰基化合物(reactive carbonyl species,RCS)主要指美拉德反应和还原糖自氧化形成的甲基乙二醛(methylglyoxal,MG)、乙二醛(glyoxal,GO)和3-脱氧葡萄糖醛酮(3-deoxyglucosone,3-DG)[1]。它们的反应活性很强,与活性氧类(reactive oxygen species,ROS)物质类似,能够同蛋白质和DNA/RNA中的氨基、巯基发生亲核加成反应,生成晚期糖基化终产物(advanced glycosylation end products,AGEs)[2]在体内引起多种病理变化[3]。其中,MG是研究最多的一种RCS,它可以由体内代谢产生,也能在食品和饮料的加工中产生并积累[4]。MG具有细胞毒性[5],并能通过与蛋白质及核酸发生非酶羰基化反应,改变蛋白质和DNA的结构和功能[6-7],引发羰基应激,诱发氧化应激与细胞凋亡[8],引起多种机体病变,如糖尿病及其并发症、代谢综合征、心血管疾病、神经退行性病变等慢性疾病和癌症[9-11]。因此,如何安全、有效地清除食品和体内的MG是一个亟待解决的关键性问题。本文介绍了食品中MG的来源与产生机理,讨论了MG及其衍生AGEs对人体的病理危害,最后总结了目前关于MG天然清除剂的体内外研究进展,为MG天然清除剂的优选及开发利用提供理论指导和参考。
1 食品中MG的产生
在食品加工与贮藏过程中,由于美拉德反应及单糖自氧化作用,MG会不断产生并积累,这是食品与饮料中MG的主要来源[12-14]。此外,酯类降解和微生物发酵过程中也会产生MG。在美拉德反应的初始阶段和发展阶段中,还原糖(通常为单糖)的醛基与蛋白质的氨基发生反应,产物脱水后形成醛亚胺,也叫西佛碱,再经Amadori分子重排形成产物果糖胺。醛亚胺和果糖胺都不稳定,继续发生分子重排并脱水形成1,2-烯醇或2,3-烯醇,再发生异构化分别生成1-脱氧葡萄糖醛酮(1-deoxyglucosone,1-DG)或3-DG。3-DG经逆向羟醛缩合反应可降解为MG。在高温下,单糖会发生自氧化形成烯醇,再经逆羟醛缩合反应裂解形成MG(图1),这一途径在含糖较高的食品加工中占主导[15]。
MG的生成与还原糖和蛋白质的种类、浓度及加工方式密切相关。葡萄糖和果糖含量高的食品中MG含量较高[15-16]。例如,高果糖玉米糖浆中MG含量为1.38~10.88 μg/mL[12],在含高果糖玉米糖浆的碳酸饮料中MG可高达139.5 μg/100 mL[13]。炒制咖啡豆[17]、麻花[14]的长期存放、油脂中多不饱和脂肪酸的氧化或高温降解[18]及葡萄的乳酸发酵[19]都会产生MG和GO。部分MG会继续和蛋白质的氨基发生反应生成AGEs,在高蛋白食品中MG衍生AGEs含量较高,并且AGEs含量随加工温度升高及时间延长而增多[20-21]。
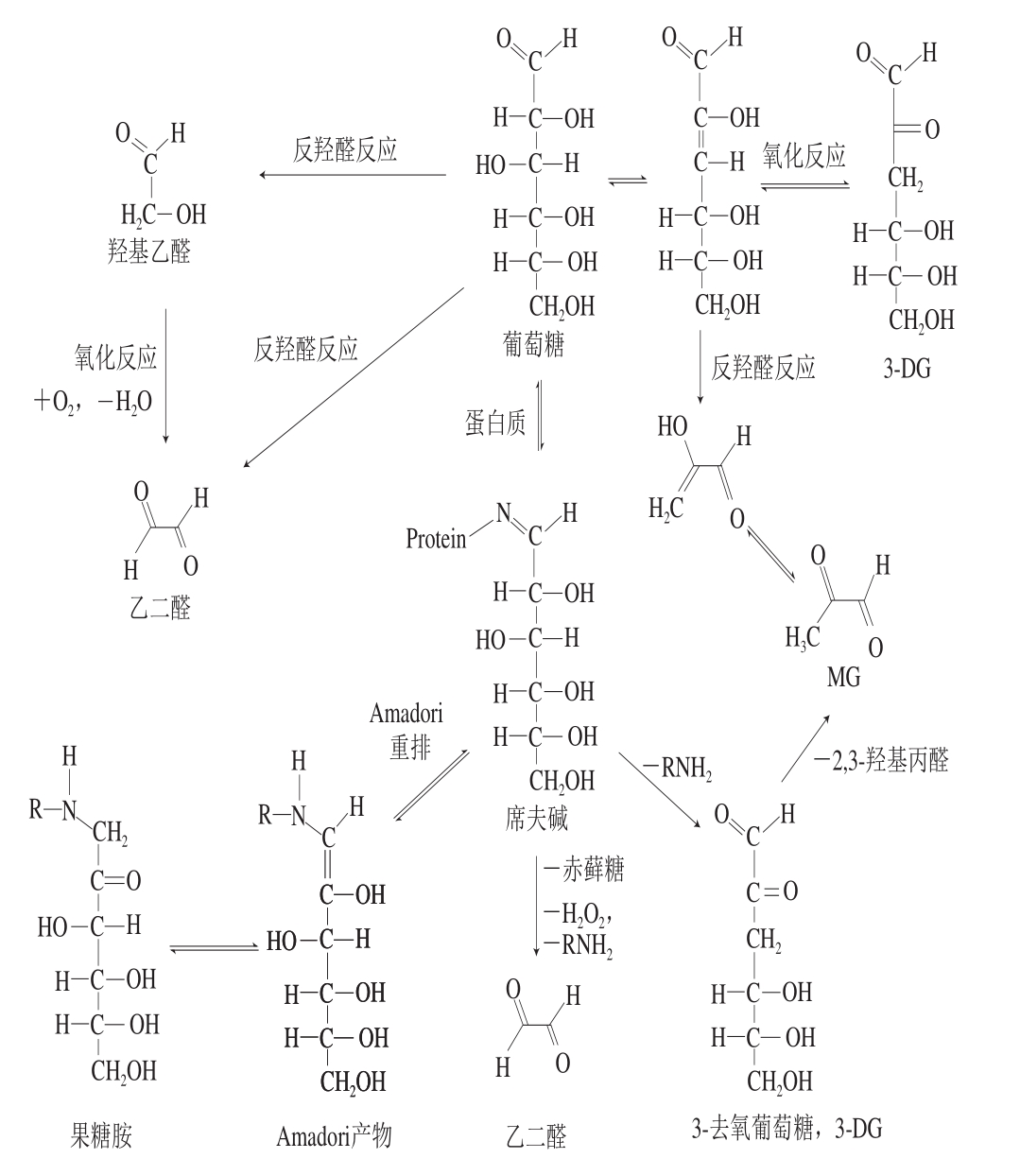
图1 MG等RCS的主要食品来源及其产生机理
Fig. 1 Major food sources and formation pathways of MG and other RCS
在体内,美拉德反应产物经Amadori重排和裂解生成MG,但机体中MG主要由糖、蛋白质和脂类的氧化分解生成,涉及一系列酶促或非酶催化反应[4]。正常生理状态下,机体内葡萄糖在糖酵解过程中由磷酸丙酮中间体经非酶催化裂解产生MG,随后被乙二醛酶系统(glyoxalase-I和glyoxalase-II)有效降解。但是在高血糖患者体内,MG的血液浓度为正常人的3~5 倍[10]。由于葡萄糖利用出现问题,致使葡萄糖、果糖、磷酸丙糖及糖化蛋白等大量积累,它们都能降解并产生MG,是血液和组织中MG水平升高的主要来源;此外,在葡萄糖利用不足时,蛋白质和脂肪也能经代谢分解产生MG[4,10]。
2 甲基乙二醛的主要糖化产物
MG是研究最多的一种1,2-二羰基化合物,它是体内AGEs形成的最重要前体[4,22],主要与蛋白质的胍基(如精氨酸)、侧链氨基(如赖氨酸)和巯基(如半胱氨酸)反应生成AGEs。MG含有2 个相邻并相互活化的羰基,反应活性很强,为葡萄糖的2万 倍[10]。MG极易与精氨酸和赖氨酸发生亲核反应,而这2 种氨基酸在蛋白质活性结构域中所占比例很高,因此体内蛋白质很容易与MG发生糖化反应[22]。此外,MG还能与DNA发生糖化反应,导致基因功能降低甚至缺失[6]。
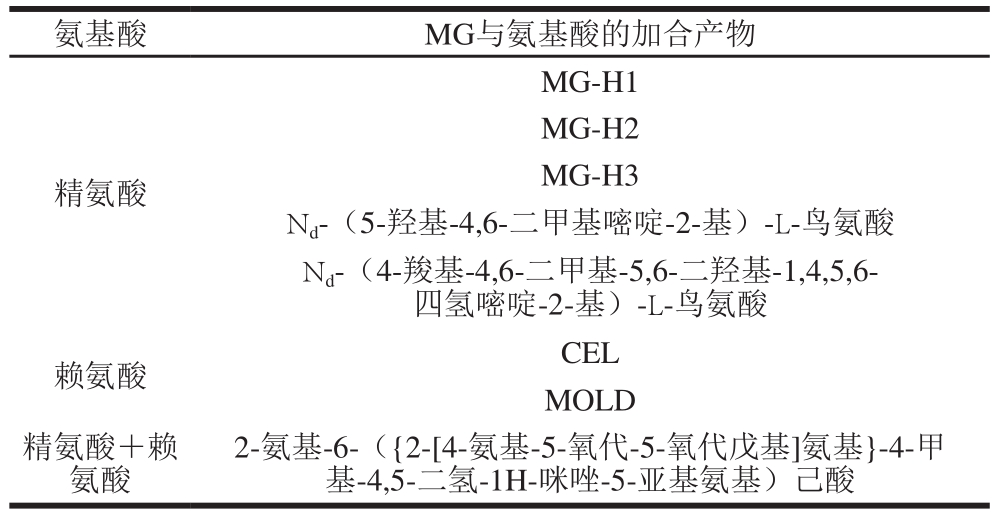
在蛋白质和核酸的糖化加合物中,研究较多的是MG与精氨酸反应形成的一类氢化咪唑酮类产物,它们环化形成3 种异构体,分别是Nd-(5-氢-5-甲基-4-咪唑酮-2-基)-L-鸟氨酸(Nd-(5-hydro-5-methyl-4-imidazolon-2-yl)-L-ornithine,MG-H1)、2-氨基-5-(2-氨基-5-氢-5-甲基-4-咪唑酮-1-基)-戊酸(2-amino-5-(2-amino-5-hydro-5-methyl-4-imidazolon-1-yl)-pentanoic acid,MG-H2)和2-氨基-5-(2-氨基-4-氢-4-甲基-5-咪唑酮-1-基)-戊酸(2-amino-5-(2-amino-4-hydro-4-methyl-5-imidazolon-1-yl)pentanoic acid,MG-H3),它们之间相互转化并处于动态平衡状态[23]。MG与赖氨酸反应生成1,3-二(Ne-赖氨酸)-4-甲基咪唑鎓(1,3-di(Ne-lysino)-4-methylimidazolium,MOLD)和Ne-1-羧乙基赖氨酸(Ne-(1-carboxyethyl)lysine,CEL),见表1。有研究表明,血浆中高浓度的MG及其糖化蛋白MG-H1和CEL的水平与糖尿病、肾病的发生发展有直接关系。因此,氢化咪唑酮类产物可以成为糖尿病、肾病早期诊断的生物标志物[24]。
表1 MG与氨基酸的主要加合产物[2,24]
Table 1 Major addition products of MG and amino acids[2,24]
3 甲基乙二醛与疾病的关系
摄入富含MG及其糖化产物(AGEs)的食物很可能对身体健康产生不良影响。身体内的MG具有细胞毒性,与蛋白质发生糖化反应,造成分子交联,引起蛋白质功能紊乱,同时生成AGEs,AGEs与糖基化终产物受体(receptor for advanced glycation endproducts,RAGE)结合,激活多条信号通路,诱导机体发生氧化应激与炎症反应,引起细胞凋亡及组织损伤。这一过程与糖尿病及其并发症[9]、代谢综合征、心血管疾病[25]、阿尔茨海默症(Alzheimer’s disease,AD)等神经退行性疾病[26]和恶性肿瘤[11,27]的发展密切相关。在此概述MG与糖尿病、神经退行性疾病和癌症的关系。
3.1 MG与糖尿病及并发症
大量动物实验证明,MG在Ⅱ型糖尿病及其并发症的发生发展中起了重要作用[28]。胰岛素是由胰腺β细胞产生的肽激素,用于调节葡萄糖的体内平衡。MG及其衍生的AGEs是造成糖尿病患者β细胞功能障碍和胰岛素抵抗的诱因之一。MG与胰岛素B链的精氨酸残基及其N-末端苯丙酸能够发生糖基化反应,使胰岛素活性降低,不能正常调节对葡萄糖的摄取[7],阻碍胰岛素信号传导,产生胰岛素抵抗[9]。AGEs通过激活其膜受体RAGE,产生氧化应激,造成炎症环境,从而损害胰岛细胞,降低胰岛素分泌[29]。研究表明,连续28 d给Sprague-Dawley大鼠背部皮下注入MG(60 mg/(kg·d))或饮水中添加1 mg/100 mL MG,导致了大鼠体内葡萄糖水平升高,胰岛素受体底物1失活,脂肪细胞中葡萄糖转运蛋白(glucose transporter 4,GLUT4)表达减少,诱导了胰岛素抵抗的发生[30-32]。食品中的MG会衍生形成AGEs,长期摄入AGEs含量高的食物会导致或加速血管疾病的发生[33],所以限制AGEs摄入量有助于改善代谢等疾病[34-35]。
MG造成的糖基化能诱导内皮功能障碍、损害微血管系统。MG与糖尿病肾病和视网膜病变密切相关[9]。由于机体循环中多数RCS和AGEs在肾脏中被过滤与清除,因而肾脏作为AGEs的主要代谢器官,易发生高血糖诱导的微血管损伤[36-37]。MG增加了不同类型肾细胞中黏附分子、促炎细胞因子和转化生长因子TGFβ的表达[38],也使肾细胞的电子呼吸链受抑制,导致线粒体功能障碍,还诱导ROS的生成并激活了转化生长因子[39]。这些因素都促使肾小球基底膜增厚和肾脏纤维化,造成糖尿病肾病[40]。由MG衍生的主要AGEs中,MG-H1是肾小球基底膜增厚的一个显著性独立预测因子,MG-H1和CEL是糖尿病肾病发展的重要早期指标[41]。血浆中的高MG含量与糖尿病肾病高患病率密切相关[42-44],通常的情况是,即便糖尿病病人的血糖水平得到了控制,蛋白质非酶糖基化和氧化应激反应仍然继续,即所谓的“高血糖记忆状态”[45]。清除MG能够有效阻断非酶糖基化反应,继而防治糖尿病并发症的发生发展,是近年来食物预防与药物治疗糖尿病并发症的研究重点之一。
3.2 MG与神经退行性疾病
大脑对能量和氧的需求量大,对氧化应激高度敏感。MG是造成氧化应激的原因之一。例如,在SH-SY5Y神经瘤母细胞中,MG处理会严重影响线粒体的呼吸及细胞能量状态,导致ROS和乳酸水平增加,造成线粒体膜电位降低和细胞内ATP水平下降[46]。在小鼠神经母细胞瘤细胞中,MG处理能促进丝裂原活化蛋白激酶(mitogenactivated protein kinase,MAPK)中JNK和p38信号传导途径激活,诱导细胞凋亡[47]。在AD中,MG能够活化GSK-3β和p38 MAPK,并调节大脑中tau的过磷酸化过程[48]。同时,在AD患者的大脑中,β-淀粉样蛋白(β-amyloid peptide,Aβ)沉积逐渐增多。在体外,Aβ与MG发生反应生成Aβ-AGEs,把Aβ-AGEs注射到SD大鼠的侧脑室中,加剧了Aβ诱导的认知损伤,还观察到RAGE的过度表达和RAGE介导的通路关键蛋白如GSK3、NF-κB、p38等的激活[49-50]。总之,增加MG的代谢和清除效率能够减轻蛋白羰基化,有助于延缓AD及衰老等慢性疾病的发生和发展。
3.3 MG与肿瘤
MG与肿瘤的关系迄今为止尚无一致的认识。一方面,MG能够抑制癌细胞的扩散和肿瘤局部生长,另一方面,研究显示MG能诱发肿瘤的形成与发展。一项于20世纪60年代的研究发现,相对于控制组,MG有效地阻止了瑞士白鼠移植腹水肉瘤的生长[51]。MG的代谢酶glyoxalase-Ⅰ在多种肿瘤组织中均存在过度表达现象,而glyoxalase-Ⅰ的过度表达使得MG被过度清除,致使MG的肿瘤抑制作用消失,肿瘤得以发展[52]。类似的研究显示,用MG清除剂处理后,能提高癌细胞对肿瘤治疗药物的敏感性[53]。相反地,用MG处理乳腺癌细胞,影响了Hsp90的伴侣活性,并降低了它与Hippo途径关键激酶大肿瘤抑制基因1的结合,使癌细胞生长和转移潜力增强[54]。MG衍生的MG-BSA能够增加人乳腺癌细胞的增殖、迁移和侵袭,表明MG-AGEs在癌症中具有促肿瘤作用[27]。与非糖尿病患者相比,糖尿病患者的癌症发病率较高,这与患者体内高MG浓度密切相关。降糖药二甲双胍具有捕获并降低糖尿病患者血浆MG水平的作用,也通过诱导细胞周期阻滞和细胞凋亡,降低各种癌细胞株的增殖,所以,二甲双胍也具有抗癌作用[55]。总之,目前学术界对MG与肿瘤的关系缺乏统一认识,癌细胞中MG的应激作用亟待探索与解释。
4 MG的清除剂研究
无论生理健康还是病理条件下,体内都会产生MG。正常情况下,机体清除MG的机制比较完善,体内MG处于正常水平;但在病理和衰老过程中,由于人体自身清除MG的效率降低,体内MG及其衍生的AGEs逐渐增多并不断积累,带来诸多疾病。在诸多RCS中,MG细胞毒性最强,但是,通过加速体内磷酸丙酮的代谢通量,或提高MG代谢酶glyoxalase-I的蛋白表达,或者摄入MG等RCS的清除剂,都能有效地降低体内MG水平。近年来研究发现,包括黄酮、二苯乙烯苷等化合物在内的植物多酚容易与MG等RCS反应,因而具有有效的MG清除作用。
4.1 食品中的MG清除剂
据报道,一些天然植物化学成分具有优良的MG清除能力,尤其是天然多酚类化合物,包括茶多酚(儿茶素、表没食子儿茶素没食子酸酯和茶黄素)[56]、苹果多酚(phloretin和phloridzin)[57]、槲皮素、芦丁、白藜芦醇[58]、石榴原花青素[59-60]、姜黄素[61]、姜辣素、姜烯醇[62]和何首乌芪[63]等。它们与MG形成加合物,相应的物质结构也都得到了鉴定。其中,茶多酚多具有高效的MG清除活性。例如,绿茶中的主要多酚表没食子儿茶素没食子酸酯能在反应5 min时捕获并清除超过90%的MG[64]。这是由于A环的C6和C8位的电子云密度高,极易与缺电子的MG发生亲核反应。清除MG的重大意义在于抑制相关AGEs的形成。体外研究一般采用牛血清白蛋白(bovine serum albumin,BSA)模拟人血浆白蛋白。例如,在模拟生理条件下,绿原酸、槲皮素和芦丁都能以剂量依赖性方式抑制MG对BSA的糖化,其中芦丁效率最高,反应1 h时对MG的捕获率可超过80%[65-66]。
4.2 MG清除剂的保护作用
采用细胞学模型对MG的细胞毒性及其可能的病理机理的研究较多,而关于天然产物对MG的毒性干预作用报道较少,作用机制也很少涉及对MG的清除作用。一些多酚具有直接保护细胞免受MG损伤的活性,机理涉及保护细胞线粒体功能损伤及改善能量代谢[67]。例如,从蜡果杨梅的根皮中分离出的杨梅黄素及二氢杨梅素,分别改善了SH-SY5Y和PC12神经细胞中由MG引起的细胞损伤,机理分别涉及到了AGEs/RAGE/NF-κB通路和AMPK/GLUT4通路,说明这类天然物质具有降低MG的细胞毒性并治疗糖尿病、脑病的潜力[68]。再如,红花的主要活性成分羟基红花黄色素A具有抗糖化作用,在10~100 μmol/L浓度范围内能够保护由MG引起的人脑微血管内皮细胞的损伤,浓度为100 μmol/L时能够抑制MG衍生AGEs的形成[69]。MG能够干扰3T3-L1脂肪细胞对葡萄糖的利用,而原花青素预处理能够起到保护细胞的作用,并能抑制CML和MG-AGEs等特定AGEs的形成[60]。有文献报道,在小鼠尿液中能够检测到大豆异黄酮与MG的2 种加合物[70]。采用绿茶多酚单体(+)-儿茶素(epicatechin,EC)灌胃16 周,改善了糖尿病db/db小鼠的肾病损伤,并在肾脏中检测到EC和MG的加合物,同时,EC也阻断了AGE引起的促炎细胞因子TNF-α和IL-1β的释放[71]。在马兜铃酸诱导的小鼠肾病模型中,肾脏MG的积累较对照组高出12 倍。采用低分子壳聚糖(500 mg/(kg∙d))喂食14 d,显著地降低了肾脏中的MG及CEL的积累(P<0.05)[72];在D-半乳糖诱导的痴呆小鼠脑中,补充齐墩果酸(0.05%~0.20%)和原儿茶酸(0.5%~2.0%)都能够剂量依赖性地预防和减轻小鼠脑中ROS和蛋白糖基化,抑制MG水平升高,降低CML水平,改善glyoxalase-I的活性和蛋白表达,抑制NF-κB p65入核及阻止炎症因子IL-1β、TNF-α和前列腺素E2的释放,具有预防和延缓衰老的功能[73-74]。
5 结 语
在食品加工和贮藏过程中,MG及其衍生物AGEs的产生与积累影响了食品的色泽和风味,更给人体健康带来诸多危害。过多的MG在人体内循环,使组织内MG含量升高,AGEs的体内生成量增加,继而加剧氧化应激、炎症发生及RAGE活性上调,从而影响人体健康,促进糖尿病及其并发症、代谢综合征、癌症、心血管疾病以及AD等神经退行性疾病的发生和发展。因此,有效地捕获和清除体内的MG对维持人体健康非常重要。对于体内MG的清除,主要有2 个机制:其一是协助和增强体内清除MG的乙醛酶系统,促进MG的快速代谢;其二是体内MG的直接捕获,即一些物质包括食品和其他天然产物在机体内与MG发生亲核反应,生成的加合物能够被代谢并排出体外,例如目前发现大多数酚类化合物都是有效的MG清除剂。总之,如何有效地清除人体内的MG是一个很重要的研究方向,而食品或其他天然产物通过直接捕获MG或者加速乙醛酶系统对MG的代谢,都是行之有效的MG清除途径。随着对天然植物(包括传统医食两用植物)在清除MG和抗羰基应激方面的深入研究,尤其是对其中有效活性成分及加合产物的鉴定和机理研究,将为清除MG功能食品的理论研究和产品开发提供理论依据,进而促进相应功能食品在人类健康领域的广泛应用。
参考文献:
[1] THORNALLEY P J, LANGBORG A, MINHAS H S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose[J]. Biochemical Journal, 1999, 344: 109-116.DOI:10.1042/0264-6021:3440109.
[2] NEMET I, VARGA-DEFTERDAROVIC L, TURK Z. Methylglyoxal in food and living organisms[J]. Molecular Nutrition and Food Research, 2006, 50(12): 1105-1117. DOI:10.1002/mnfr.200600065.
[3] CAI Weijing, RAMDAS M, ZHU Li, et al. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1[J].Proceedings of the National Academy of Sciences, 2012, 109(39):15888-15893. DOI:10.1073/pnas.1205847109.
[4] THORNALLEY P J. Pharmacology of methylglyoxal: formation,modification of proteins and nucleic acids, and enzymatic detoxification-a role in pathogenesis and antiproliferative chemotherapy[J]. General Pharmacology, 1996, 27(4): 565-573.DOI:10.1016/0306-3623(95)02054-3.
[5] CHAKRABORTY S, KARMAKAR K, CHAKRAVORTTY D.Cells producing their own nemesis: understanding methylglyoxal metabolism[J]. IUBMB Life, 2014, 66(10): 667-678. DOI:10.1002/iub.1324.
[6] MURATAKAMIYA N, KAMIYA H. Methylglyoxal, an endogenous aldehyde, crosslinks DNA polymerase and the substrate DNA[J]. Nucleic Acids Research, 2001, 29(16): 3433-3438. DOI:10.1093/nar/29.16.3433.
[7] JIA Xuming, OLSON J H D, ROSS R S A, et al. Structural and functional changes in human insulin induced by methylglyoxal[J]. The FASEB Journal, 2006, 20(9): 1555-1557. DOI:10.1096/fj.05-5478fje.
[8] RABBANI N, THORNALLEY J P. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease[J]. Biochemical and Biophysical Research Communications, 2015, 458(2): 221-226.DOI:10.1097/00003246-199507000-00010.
[9] MATAFOME P, SENA C, SEICA R. Methylglyoxal, obesity, and diabetes[J]. Endocrine, 2013, 43(3): 472-484. DOI:10.1007/s12020-012-9795-8.
[10] VAN NGUYEN C. Toxicity of the AGEs generated from the Maillard reaction: on the relationship of food-AGEs and biological-AGEs [J].Molecular Nutrition and Food Research, 2006, 50(12): 1140-1149.DOI:10.1002/mnfr.200600144.
[11] LIN J A, WU C H, LU C C, et al. Glycative stress from advanced glycation end products (AGEs) and dicarbonyls: an emerging biological factor in cancer onset and progression[J]. Molecular Nutrition & Food Research, 2016, 60(8): 1850-1864. DOI:10.1002/mnfr.201500759.
[12] GENSBERGER S, MITTELMAIER S, GLOMB MA, et al.Identification and quantification of six major α-dicarbonyl process contaminants in high-fructose corn syrup[J]. Analytical and Bioanalytical Chemistry, 2012, 403(10): 2923-2931. DOI:10.1007/s00216-012-5817-x.
[13] LO Chiyu, LI Shiming, WANG Yu, et al. Reactive dicarbonyl compounds and 5-(hydroxymethyl)-2-furfural in carbonated beverages containing high fructose corn syrup[J]. Food Chemistry, 2008, 107(3):1099-1105. DOI:10.1016/j.foodchem.2007.09.028.
[14] LIU Huicui, LI Juxiu. Changes in glyoxal and methylglyoxal content in the fried dough twist during frying and storage[J]. European Food Research and Technology, 2014, 238(2): 323-331. DOI:10.1007/s00217-013-2110-y.
[15] ALVAREZ-SUAREZ J M, GIAMPIERI F, BATTINO M. Honey as a source of dietary antioxidants: structures, bioavailability and evidence of protective effects against human chronic diseases[J]. Current Medicinal Chemistry, 2013, 20(5): 621-638.DOI:10.2174/092986713804999358.
[16] ZHANG Dongmei, JIAO Ruiqing, KONG Lingdong. High dietary fructose: direct or indirect dangerous factors disturbing tissue and organ functions[J]. Nutrients, 2017, 9(4): 335-359. DOI:10.3390/nu9040335.
[17] WANG J, CHANG T J. Methylglyoxal content in drinking coffee as a cytotoxic factor[J]. Journal of Food Science, 2010, 75(6): 167-171.DOI:10.1111/j.1750-3841.2010.01658.x.
[18] KAZUTOSHI F, TAKAYUKI S. Formation of genotoxic dicarbonyl compounds in dietary oils upon oxidation[J]. Lipids, 2004, 39(5): 481-486. DOI:10.1007/s11745-004-1254-y.
[19] SANTOS C M, VALENTE I M, GONCALVES L M, et al.Chromatographic analysis of methylglyoxal and other alpha-dicarbonyls using gas-diffusion microextraction[J]. Analyst,2013, 138(23): 7233-7237. DOI:10.1039/c3an00766a.
[20] 房红娟, 王丽娟, 张双凤, 等. 高蛋白食品加工模拟体系中晚期糖基化末端产物的形成[J]. 中国食品学报, 2014, 14(2): 28-34.DOI:10.13982/j.mfst.1673-9078.2014.12.006.
[21] GOLDBERG T, CAI Weijing, PEPPA M, et al. Advanced glycoxidation end products in commonly consumed foods[J]. Journal of the American Dietetic Association, 2004, 104(8): 1287-1291.DOI:10.1016/j.jada.2004.05.214.
[22] RABBANI N, THORNALLEY P J. The critical role of methylglyoxal and glyoxalase 1 in diabetic nephropathy[J]. Diabetes, 2014, 63(1): 50-52. DOI:10.2337/db13-1606.
[23] RABBANI N, THORNALLEY P J. Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome[J]. Amino Acids, 2012, 42(4): 1133-1142.DOI:10.1007/s00726-010-0783-0.
[24] BEISSWENGER J P, HOWELL K S, RUSSELL G, et al. Detection of diabetic nephropathy from advanced glycation endproducts(AGEs) differs in plasma and urine, and is dependent on the method of preparation[J]. Amino Acids, 2014, 46(2): 311-319. DOI:10.1007/s00726-013-1533-x.
[25] HANSSEN N M, WOUTERS K, HUIJBERTS M S, et al. Higher levels of advanced glycation endproducts in human carotid atherosclerotic plaques are associated with a rupture-prone phenotype[J]. European Heart Journal, 2014, 35(17): 1137-1146.DOI:10.1093/eurheartj/eht402.
[26] ANGELONI C, ZAMBONIN L, HRELIA S. Role of methylglyoxal in Alzheimer’s disease[J]. BioMed Research International, 2014, 2014:1-12. DOI:10.1155/2014/238485.
[27] SHARAFA1 H, MATOU-NASRIB1 S, WANGA Q, et al. Advanced glycation endproducts increase proliferation, migration and invasion of the breast cancer cell line MDA-MB-231[J]. Biochimica et Biophysica Acta: Molecular Basis of Disease, 2015, 1852(3): 429-441.DOI:10.1016/j.bbadis.2014.12.009.
[28] SIREESH D, BHAKKIYALAKSHMI E, PONJAYANTHI B, et al. Pathophysiological insights of methylglyoxal induced type-2 diabetes[J]. Chemical Research in Toxicology, 2015, 28(9): 1666-1674. DOI:10.1021/acs.chemrestox.5b00171.
[29] LUIS M A O, ANA L, RICARDO A G, et al. Insulin glycation by methylglyoxal results in native-like aggregation and inhibition of fibril formation[J]. BMC Biochemistry, 2011, 12(1): 41-52.DOI:10.1186/1471-2091-12-41.
[30] JIA Xuming, WU Lingyun. Accumulation of endogenous methylglyoxal impaired insulin signaling in adipose tissue of fructose-fed rats[J]. Molecular and Cellular Biochemistry, 2007, 306(1/2): 133-139.DOI:10.1007/s11010-007-9563-x.
[31] DHAR A, DHAR I, JIANG Bo, et al. Chronic methylglyoxal infusion by minipump causes pancreatic β-cell dysfunction and induces type 2 diabetes in sprague-dawley rats[J]. Diabetes, 2011, 60(3): 899-908.DOI:10.2337/db10-0627.
[32] GUO Qi, MORI T, JIANG Yue, et al. Methylglyoxal contributes to the development of insulin resistance and salt sensitivity in Sprague-Dawley rats[J]. Journal of Hypertension, 2009, 27(8): 1664-1671. DOI:10.1097/HJH.0b013e32832c419a.
[33] URIBARRI J, CAI Weijing, WOODWARD M, et al. Elevated serum advanced glycation endproducts in obese indicate risk for the metabolic syndrome: a link between healthy and unhealthy obesity?[J].Journal of Clinical Endocrinology and Metabolism, 2015, 100(5):1957-1966. DOI:10.1210/jc.2014-3925.
[34] URIBARRI J, CAI Weijing, RAMDAS M, et al. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of ager1 and sirt1[J]. Diabetes Care,2011, 34(7): 1610-1616. DOI:10.2337/dc11-0091.
[35] DE COURTEN B, DE COURTEN M P, SOLDATOS G, et al. Diet low in advanced glycation end products increases insulin sensitivity in healthy overweight individuals: a double-blind, randomized, crossover trial[J]. American Journal of Clinical Nutrition, 2016, 103(6): 1426-1433. DOI:10.3945/ajcn.115.125427.
[36] NEGRESALVAYRE A, SALVAYRE R, AUGÉ N, et al.Hyperglycemia and glycation in diabetic complications[J].Antioxidants & Redox Signaling, 2009, 11(12): 3071-3109.DOI:10.1089/ars.2009.2484.
[37] NAKAJOU K, HORIUCHI S, SAKAI M, et al. Renal clearance of glycolaldehyde- and methylglyoxal- modified proteins in mice is mediated by mesangial cells through a class A scavenger receptor(SR-A)[J]. Diabetologia, 2005, 48(2): 317-327. DOI:10.1007/s00125-004-1646-6.
[38] ZHAO Yanhua, BANERJEE S, LEJEUNE W S, et al. NF-κB-inducing kinase increases renal tubule epithelial inflammation associated with diabetes[J]. Journal of Diabetes Research, 2011, 2011:192564. DOI:10.1155/2011/192564.
[39] ROSCA M, MUSTATA T, KINTER M, et al. Glycation of mitochondrial proteins from diabetic rat kidney is associated with excess superoxide formation[J]. American Journal of Physiology Renal Physiology, 2005, 289(2): F420-F430. DOI:10.1152/ajprenal.00415.2004.
[40] TANG S C W, CHAN L Y Y, LEUNG J C K, et al. Differential effects of advanced glycation end-products on renal tubular cell inflammation[J]. Nephrology, 2011, 16(4): 417-425. DOI:10.1111/j.1440-1797.2010.01437.x.
[41] BEISSWENGER P J, HOWELL S K, RUSSELL G B, et al. Early progression of diabetic nephropathy correlates with methylglyoxalderived advanced glycation end products[J]. Diabetes Care, 2013,36(10): 3234-3239. DOI:10.2337/dc12-2689.
[42] LU Jianxin, RANDELL E, HAN Yingchun, et al. Increased plasma methylglyoxal level, inflammation, and vascular endothelial dysfunction in diabetic nephropathy[J]. Clinical Biochemistry, 2011,44(4): 307-311. DOI:10.1016/j.clinbiochem.2010.11.004.
[43] NAKAYAMA K, NAKAYAMA M, IWABUCHI M, et al. Plasma alpha-oxoaldehyde levels in diabetic and nondiabetic chronic kidney disease patients[J]. American Journal of Nephrology, 2008, 28(6):871-878. DOI:10.1159/000139653.
[44] BEISSWENGER P J, DRUMMOND K S, NELSON R G, et al.Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress[J]. Diabetes, 2005, 54(11): 3274-3281. DOI:10.2337/diabetes.54.11.3274.
[45] PANENI F, COSENTINO F. Advanced glycation end products and plaque instability: a link beyond diabetes[J]. European Heart Journal,2014, 35(17): 1095-1097. DOI:10.1093/eurheartj/eht454.
[46] DE ARRIBA S G, STUCHBURY G, YARIN J, et al. Methylglyoxal impairs glucose metabolism and leads to energy depletion in neuronal cells-protection by carbonyl scavengers[J]. Neurobiology of Aging,2007, 28(7): 1044-1050. DOI:10.1016/j.neurobiolaging.2006.05.007.
[47] HUANG Shangming, CHUANG Hongchin, WU Chihao, et al.Cytoprotective effects of phenolic acids on methylglyoxal-induced apoptosis in Neuro-2A cells[J]. Molecular Nutrition and Food Research, 2008, 52(8): 940-949. DOI:10.1002/mnfr.200700360.
[48] LI Xiaohong, XIE Jiazhao, XIA Jiang, et al. Methylglyoxal induces tau hyperphosphorylation via promoting AGEs formation[J].Neuromolecular Medicine, 2012, 14(4): 338-348. DOI:10.1007/s12017-012-8191-0.
[49] CHEN Chong, LI Xiaohong, TU Yue, et al. Abeta-AGE aggravates cognitive deficit in rats via RAGE pathway[J]. Neuroscience, 2014,257: 1-10. DOI:10.1016/j.neuroscience.2013.10.056.
[50] LI Xiaohong, DU Lailing, CHENG Xiangshu, et al. Glycation exacerbates the neuronal toxicity of β-amyloid[J]. Cell Death &Disease, 2013, 4: e673. DOI:10.1038/cddis.2013.180.
[51] EGYÜD L G, SZENT-GYÖRGYI A. Cancerostatic action of methylglyoxal[J]. Science, 1968, 160: 1140. DOI:10.1126/science.160.3832.1140.
[52] 王蕾, 朱迪娜, 吕翠, 等. 甲基乙二醛与相关疾病的研究进展[J]. 中国药理学通报, 2014, 30(4): 456-459. DOI:10.3969/j.issn.1001-1978.2014.04.004.
[53] SAKAMOTO H, MASHIMA T, YAMAMOTO K, et al. Modulation of heat-shock protein 27 (Hsp27) anti-apoptotic activity by methylglyoxal modification[J]. Journal of Biological Chemistry, 2002,277: 45770-45775. DOI:10.1074/jbc.M207485200.
[54] NOKIN M J, DURIEUX F, PEIXOTO P, et al. Methylglyoxal, a glycolysis side-product, induces Hsp90 glycation and YAP-mediated tumor growth and metastasis[J]. eLife, 2016, 5: e19375. DOI:10.7554/eLife.19375.
[55] SAHRA I B, LAURENT K, LOUBAT A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level[J]. Oncogene, 2008, 27(25): 3576-3586.DOI:10.1038/sj.onc.1211024.
[56] LO C Y, LI S J, TIAN D, et al. Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions[J].Molecular Nutrition and Food Research, 2006, 50(12): 1118-1128.DOI:10.1002/mnfr.200600094.
[57] SHAO Xi, BAI Naisheng, HE Kan, et al. Apple polyphenols, phloretin and phloridzin: new trapping agents of reactive dicarbonyl species[J].Chemical Research in Toxicology, 2008, 21(10): 2042-2050.DOI:10.1021/tx800227v.
[58] SHEN Yixiao, XU Zhimin, SHENG Zhanxu. Ability of resveratrol to inhibit advanced glycation end product formation and carbohydrate-hydrolyzing enzyme activity, and to conjugate methylglyoxal[J]. Food Chemistry, 2017, 216: 153-160. DOI:10.1016/j.foodchem.2016.08.034.
[59] KUMAGAI Y, NAKATANI S, ONODERA H, et al. Anti-glycation effects of pomegranate (Punica granatum L.) fruit extract and its components in vivo and in vitro[J]. Journal of Agricultural and Food Chemistry, 2015, 63(35): 7760-7764. DOI:10.1021/acs.jafc.5b02766.
[60] LIU Weixi, MA Hang, FROST L, et al. Pomegranate phenolics inhibit formation of advanced glycation endproducts by scavenging reactive carbonyl species[J]. Food and Function, 2014, 5(11): 2996-3004.DOI:10.1039/c4fo00538d.
[61] HU Teyu, HU Miaolin, CHYAU Charngcherng, et al. Trapping of methylglyoxal by curcumin in cell-free systems and in human umbilical vein endothelial cells[J]. Journal of Agricultural and Food Chemistry, 2012, 60(33): 8190-8196. DOI:10.1021/jf302188a.
[62] WEI Yan, HAN Chanshuai, WANG Yujing, et al. Ribosylation triggering alzheimer’s disease-like tau hyperphosphorylation via activation of camkii[J]. Aging Cell, 2015, 14(5): 754-763.DOI:10.1111/acel.12355.
[63] LÜ Lishuang, SHAO Xi, WANG Liyan, et al. Stilbene glucoside from polygonum multiflorum thunb: a novel natural inhibitor of advanced glycation end product formation by trapping of methylglyoxal[J].Journal of Agricultural and Food Chemistry, 2010, 58(4): 2239-2245.DOI:10.1021/jf904122q.
[64] SANG Shengmin, SHAO Xi, BAI Naisheng, et al. Tea polyphenol(−)-epigallocatechin-3-gallate: a new trapping agent of reactive dicarbonyl species[J]. Chemical Research in Toxicology, 2007, 20(12):1862-1870. DOI:10.1021/tx700190s.
[65] YOON S R, SHIM S M. Inhibitory effect of polyphenols in houttuynia cordata on advanced glycation end-products (AGEs) by trapping methylglyoxal[J]. LWT-Food Science and Technology, 2015, 61(1):158-163. DOI:10.1016/j.lwt.2014.11.014.
[66] 李晓明, 邓荣华, 孔阳辉, 等. 芦丁抑制牛血清白蛋白糖基化[J]. 食品科学, 2014, 35(3): 85-89. DOI:10.7506/spkx1002-6630-201403018.
[67] WANG Yuehua, YU Haitao, PU Xiaoping, et al. Myricitrin alleviates methylglyoxal-induced mitochondrial dysfunction and AGEs/RAGE/NF-κB pathway activation in SH-SY5Y cells[J]. Journal of Molecular Neuroscience, 2014, 53(4): 562-570. DOI:10.1007/s12031-013-0222-2.
[68] JIANG Baoping, LE Le, PAN Huimin, et al. Dihydromyricetin ameliorates the oxidative stress response induced by methylglyoxal via the AMPK/GLUT4 signaling pathway in PC12 cells[J]. Brain Research Bulletin, 2014, 109: 117-126. DOI:10.1016/j.brainresbull.2014.10.010.[69] LI Wenlu, LIU Jie, HE Ping, et al. Hydroxysafflor yellow a protects methylglyoxal-induced injury in the cultured human brain microvascular endothelial cells[J]. Neuroscience Letters, 2013, 549:146-150. DOI:10.1016/j.neulet.2013.06.007.
[70] WANG Pei, CHEN Huadong, SANG Shengmin. Trapping of methylglyoxal by genistein and its metabolites in mice[J]. Chemical Research in Toxicology, 2016, 29(3): 406-414. DOI:10.1021/acs.chemrestox.5b00516.
[71] ZHU Dina, WANG Lei, ZHOU Qile, et al. (+)-Catechin ameliorates diabetic nephropathy by trapping methylglyoxal in type 2 diabetic mice[J]. Molecular Nutrition and Food Research, 2014, 58(12): 2249-2260. DOI:10.1002/mnfr.201400533.
[72] CHOU C K, CHEN S M, LI Y C, et al. Low-molecular-weight chitosan scavenges methylglyoxal and Nε-(carboxyethyl)lysine, the major factors contributing to the pathogenesis of nephropathy[J].SpringerPlus, 2015, 4: 312. DOI:10.1186/s40064-015-1106-4.
[73] TSAI S J, YIN M C. Anti-oxidative, anti-glycative and anti-apoptotic effects of oleanolic acid in brain of mice treated by D-galactose[J].European Journal of Pharmacology, 2012, 689(1/2/3): 81-88.DOI:10.1016/j.ejphar.2012.05.018.
[74] TSAI S J, YIN M C. Anti-glycative and anti-inflammatory effects of protocatechuic acid in brain of mice treated by D-galactose[J]. Food and Chemical Toxicology, 2012, 50(9): 3198-3205. DOI:10.1016/j.fct.2012.05.056.