秀丽隐杆线虫模型在食品营养评价中的应用研究进展
杨 番1,夏程程1,钟晓凌1,李 琴1,李 茜1,张智源2,史文博3,徐 宁1,吴 茜1,胡 勇1,柳志杰1,汪 超1,周梦舟1,*
(1.湖北工业大学 工业发酵湖北省协同创新中心,湖北省食品发酵工程技术研究中心,湖北 武汉 430068;2.武汉市环境检测中心,湖北 武汉 430015;3.湖北省阿克瑞德检验检测有限公司,湖北 武汉 430077)
摘 要:食品营养一直是食品领域研究的重点和热点,近些年消费者对功能性食品的消费量逐年增加,而对食品的功能性评价需要模式生物来完成。秀丽隐杆线虫作为模式生物,具有信号通路同人类相似的优点,且成本低、易于培养。本文综述了秀丽隐杆线虫与人类信号通路的同源性,对食品中活性因子的营养功能进行分类评价,并且对秀丽隐杆线虫在食品营养评价中的研究进展进行总结,为食品营养研究以及功能性食品的研发提供参考。
关键词:食品营养;秀丽隐杆线虫;信号通路;营养评价
食品营养逐步引起国民的重视,市场上各种功能性食品、营养素应运而生。长期以来,科研人员利用小鼠、狗、猩猩等动物模型进行营养评价研究。动物模型在很大程度上提高了人体食用新型功能性食品和药品的安全性,能够对功能性食品的营养功能进行评价,同时为证明营养素是否对慢性疾病具有一定功效提供理论支撑。然而对动物模型的使用一直存在争议,动物保护主义者认为,利用活的生物进行实验,对动物而言是一种非人道主义的折磨。同时,哺乳动物模型还存在一些问题,如实验周期较长、成本较高。秀丽隐杆线虫这种无脊椎真核生物为营养评价提供了另一种选择。在低等生物和高等生物中,基因的结构和功能是相似的。用较易研究的生物作为模型,研究其基因的结构和生物学功能,由此获得的信息不仅可以类比于其他较难研究的生物,还可用于预测相似的人体基因的功能[1]。
秀丽隐杆线虫(Caenorhabditis elegans)作为模式生物具有易于在实验室饲养、世代短、子代多的优点,同时其肠细胞与人类相似,因此是一种较为理想的模式生物。本文以秀丽隐杆线虫为模型,对食品中活性因子的营养功能进行分类,并对秀丽隐杆线虫在食品营养评价研究中的进展进行总结,为食品营养评价以及功能性食品的研发提供依据。
1 利用秀丽隐杆线虫作为模式生物的优势
秀丽隐杆线虫以细菌为食物,体长通常为1 mm,通身透明、易于观察,对人类以及环境没有危害。1963年,英国的Brenner首次发现了秀丽隐杆线虫成虫是研究发育生物学和神经生物学的理想模式动物[1]。经历了近半个世纪,秀丽隐杆线虫已成为研究细胞凋亡、神经发育和学习记忆等多种复杂生命现象调控机制及环境化学品毒性评价的重要模式生物之一[2]。
1.1 易于培养
在实验室中通常选用NGM(nematode growth media)培养基,用大肠杆菌(OP50)进行平板涂布喂食,使秀丽隐杆线虫生长繁殖。秀丽隐杆线虫在生化培养箱20 ℃下平均寿命约为2~3 周,产卵后长成成虫只需要3~4 d。同时每只雌雄同体的成虫在3~4 d内大约可以产卵400 个,进行线虫同期化处理后即可进行实验[3]。相对于其他模式生物而言,秀丽隐杆线虫培养成本较低、占地面积相对较少、生长繁殖快且易于实验室操作。
1.2 易于观察研究
对秀丽隐杆线虫肠道的研究得益于其机体的透明性[4],从而可以看到虫体的内部组织。秀丽隐杆线虫对遗传操作的适应性使得研究人员可以使用荧光标记,如绿色荧光蛋白构建转录和翻译[5]。因此,微生物在秀丽隐杆线虫体内可以实时显现。
2 在食品营养方面秀丽隐杆线虫与人类信号通路的同源性
1998年人们已完成对秀丽隐杆线虫的基因组测序,其基因与人类基因的同源性达40%[6]。研究表明,秀丽隐杆线虫是一种多细胞真核生物,保守估计约有65%的基因与人类疾病相关[7],同时,其已被证明有12 条信号通路与人体的信号通路相同[8],其中大部分信号通路都与营养代谢密切相关(表1)。
表1 在食品营养方面秀丽隐杆线虫与人类的同源信号通路
Table 1 Homologous signaling pathways of Caenorhabditis elegans and humans
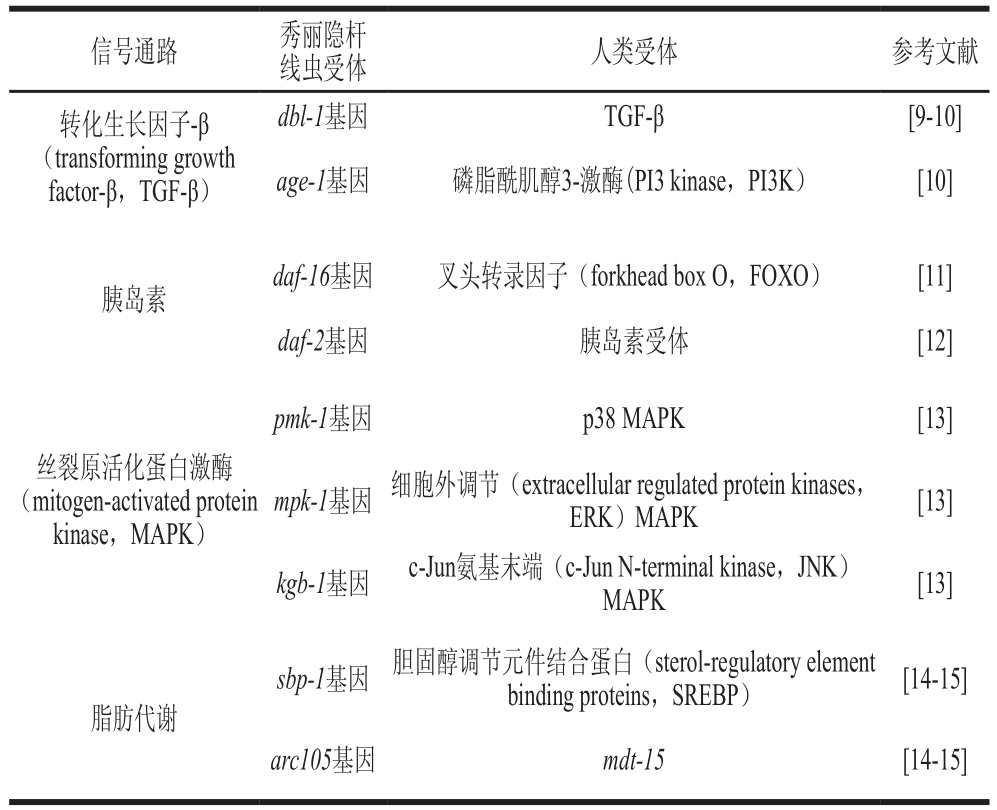
信号通路 秀丽隐杆线虫受体 人类受体 参考文献转化生长因子-β(transforming growth factor-β,TGF-β)dbl-1基因 TGF-β [9-10]age-1基因 磷脂酰肌醇3-激酶(PI3 kinase,PI3K) [10]胰岛素 daf-16基因 叉头转录因子(forkhead box O,FOXO) [11]daf-2基因 胰岛素受体 [12]丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)pmk-1基因 p38 MAPK [13]mpk-1基因 细胞外调节(extracellular regulated protein kinases,ERK)MAPK [13]kgb-1基因 c-Jun氨基末端(c-Jun N-terminal kinase,JNK)MAPK [13]脂肪代谢sbp-1基因 胆固醇调节元件结合蛋白(sterol-regulatory element binding proteins,SREBP) [14-15]arc105基因 mdt-15 [14-15]
2.1 胰岛素信号通路
生物学界关于衰老遗传学的知识和信息大部分来源于对秀丽隐杆线虫的研究[16]。由于人与许多哺乳动物以及秀丽隐杆线虫均具有daf-16基因,同时胰岛素信号通路高度保守[17],因此对秀丽隐杆线虫胰岛素信号通路的研究有助于更好地了解人类衰老以及食品营养对寿命的影响。
daf-16在体内大多数细胞中十分活跃,与人体内FOXO家族调节基因具有同源性[18]。研究发现,daf-16的活性与秀丽隐杆线虫的寿命具有关联性,daf-16的活性越高其寿命越长[19]。daf-2信号通路是线虫中最具有代表性同时也是研究的最透彻的两条信号通路之一[20]。daf-2是胰岛素生长因子信号通路中的单一受体,主要通过隔离细胞质中的叉头转录因子daf-16缩短秀丽隐杆线虫的寿命[21]。daf-2与胰岛素受体有36%的同源性,与胰岛素样生长因子有35%的同源性[22]。daf-2的羧基末端有一个与哺乳动物胰岛素受体底物相似的功能区域[23],有同源的自动磷酸化区域,在人胰岛素受体中,自动磷酸化对蛋白酪氨酸激酶的活性发挥正调控作用[24]。
2.2 MAPK信号通路
p38 MAPK、ERK MAPK、JNK MAPK是3 条重要的MAPK信号传导途径[25]。分别对应于秀丽隐杆线虫中的pmk-1、mpk-1和kgb-1信号途径,其中pmk-1在抗病原菌的免疫应激中发挥重要作用[26]。MAPKKK、MAPKK、MAPK 3 种激酶依次激活,共同调节细胞的生长、分化、炎症反应等多种重要的细胞生理过程[27]。
2.3 TGF-β信号通路
研究表明,dbl-1基因是TGF-β在秀丽隐杆线虫中的同源基因[28],与形体大小以及生殖系统相关,同时具有免疫调节抵抗病原菌的作用[29]。经典的TGF-β信号通路广泛地参与了秀丽隐杆线虫的各项生理活动,包括休眠态幼虫的形成、脂肪代谢、进食速率、寿命调节等[30]。TGF-β信号通路在秀丽隐杆线虫抗铜绿假单胞菌的逃避行为中发挥正调控作用。因此,可以通过对TGF-β通路的免疫调节抵抗致病菌来了解、鉴别动物体中的致病菌,以及其抵抗致病菌的作用机制[31]。
3 秀丽隐杆线虫在营养评价研究中的应用
3.1 抗衰老研究
衰老属于自然退化的过程,主要表现为机体的功能退化和免疫力降低,最终导致机体死亡[32]。在秀丽隐杆线虫中发现了第1个衰老相关基因age-1和衰老相关通路IIS(胰岛素/胰岛素生长因子-1信号通路)[33]。IIS通路是已知的调控生物体发育和衰老的最重要的信号通路[34],具有高度的保守性,同时已被证明与人类具有相似的衰老进程。大量研究表明多酚类化合物均具有抗衰老的作用。Kim等[35]发现原儿茶酸可以延长秀丽隐杆线虫的寿命,添加200 μmol/L原儿茶酸的秀丽隐杆线虫平均寿命比对照组延长5 d。Wood等[36]发现,用白藜芦醇喂食秀丽隐杆线虫可以延长其寿命,其是通过激活sir-2.1基因完成的。Chen Wei等[37]发现松果菊苷可以显著延长秀丽隐杆线虫的寿命,喂食200 μmol/L松果菊苷的秀丽隐杆线虫平均寿命延长了13.64%。Fei Tianyi等[38]发现茶水提取物可以延长秀丽隐杆线虫的寿命,用灵芝水提取物作为阳性对照,正常培养基作为阴性对照,3 种浓度的红茶、普洱茶和绿茶提取物均能显著延长秀丽隐杆线虫的寿命。Taira等[39]发现冲绳蜂胶可以显著延长秀丽隐杆线虫的寿命,hsp-16.2基因在秀丽隐杆线虫中被pak-1阻断失活,是寿命和抗压力、热冲击性能的关键调节因子之一,可作为预测寿命的压力敏感指标。添加0.5~1.0 μg/mL冲绳蜂胶可以使hsp-16.2基因表达上调(31.0±0.7)%;添加1 μg/mL冲绳蜂胶喂食秀丽隐杆线虫,其寿命与对照组相比延长了约33%,且冲绳蜂胶的效果明显强于阳性对照白藜芦醇。
大量研究表明,秀丽隐杆线虫寿命的延长可能取决于daf-16基因。Zheng Shanqing等[40]发现绿原酸可能通过胰岛素/类胰岛素生长因子信号通路调控下游daf-16基因以延长秀丽隐杆线虫寿命,与空白对照组相比,用50 μmol/L绿原酸处理的秀丽隐杆线虫平均寿命延长了23.3%,最大寿命从20 d延长到25 d;受daf-16基因调控的下游基因sod-3、dod-3、hsp-12.6、hsp-16.1和hsp-16.2在野生型秀丽隐杆线虫中表达均上调,绿原酸处理的秀丽隐杆线虫寿命延长取决于daf-16。刘冰冰[41]研究了铁皮石斛多糖对秀丽隐杆线虫寿命的影响。与对照组相比,铁皮石斛多糖能够显著延长秀丽隐杆线虫的寿命,使其daf-16、hsp-16.2基因表达上调,其作用机制可能是通过胰岛素信号通路调控下游daf-16基因完成对秀丽隐杆线虫耐受能力的提高。Meng Fanhui等[42]发现添加3.94 mg/mL更年春可以显著延长秀丽隐杆线虫的寿命,这取决于age-1和daf-16基因。Liao等[43]发现喂食秀丽隐杆线虫不同浓度的姜黄素可以有效延长其寿命,其通过延长daf-16突变体寿命以达到抗衰老能力。
也有研究表明,秀丽隐杆线虫寿命的延长取决于skn-1。Havermann等[44]发现黄芩素对秀丽隐杆线虫寿命的延长与激活skn-1而不是daf-16有关。用100 μmol/L黄芩素喂食秀丽隐杆线虫,其平均寿命和最大寿命分别延长57%和24%,而对于敲除skn-1的秀丽隐杆线虫黄芩素延长寿命的效应完全消失。Dehghan等[45]发现肼苯哒嗪通过skn-1延长秀丽隐杆线虫寿命,在含有10~100 μmol/L肼苯哒嗪的培养基上培养同步的野生型秀丽隐杆线虫,100 μmol/L肼苯哒嗪对秀丽隐杆线虫寿命影响最大。肼苯哒嗪处理显著增加了带有绿色荧光蛋白标记的skn-1基因在肠细胞核中的定位,同时其延长寿命效应在敲除skn-1的秀丽隐杆线虫和skn-1(zu135)突变体中完全丧失。由于衰老的终点是死亡,秀丽隐杆线虫的生命周期相对较短,关于秀丽隐杆线虫抗衰老的研究大部分是对其寿命的研究。
从以上的研究结果可以看出,具有抗衰老能力的食品活性成分一般是通过胰岛素信号通路调节daf-16和激活skn-1实现的。如图1所示,胰岛素与daf-2/胰岛素受体相互作用以激活age-1/PI3K并调节多个下游基因的活性[46],包括daf-16/FOXO和skn-1转录因子,提升秀丽隐杆线虫抗衰老的能力。
3.2 抗氧化性研究
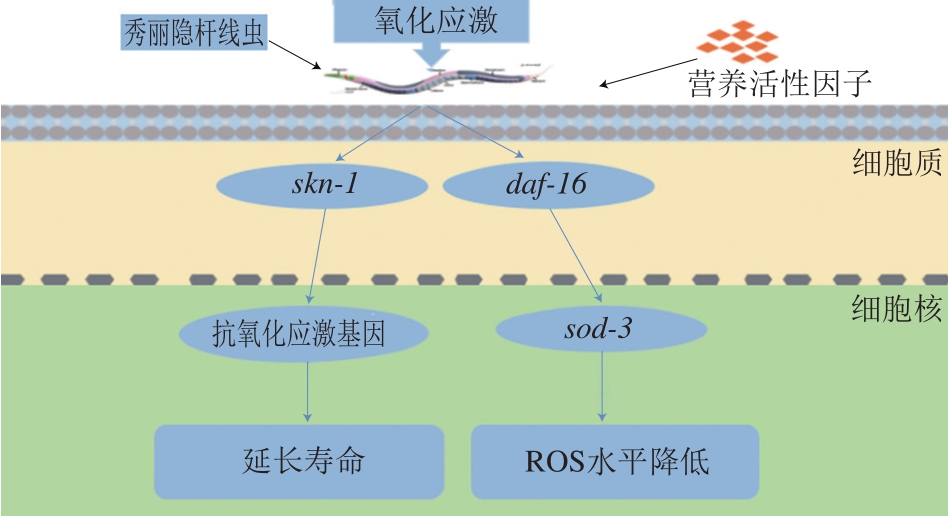
氧化应激源于人类对衰老的认识,指机体遭受有害的刺激时产生过多的高分子活性成分自由基,所产生的活性氧(reactive oxygen species,ROS)自由基超出了自身的清除能力,氧化系统和抗氧化系统失衡,从而导致机体组织损伤[47]。秀丽隐杆线虫的氧化应激应答模型包括百草枯、胡桃醌、亚砷酸盐、H2O2等。秀丽隐杆线虫体内有两类主要的抗氧化酶:超氧化物歧化酶(superoxide dismutase,SOD)和过氧化氢酶(catalase,CAT)[48]。这两类抗氧化酶具有清除超氧化物自由基和H2O2的功能。当秀丽隐杆线虫体内的抗氧化酶难以抵挡自由基的进攻从而引起机体组织功能损伤时[49],就需要补充外源的抗氧化剂清除体内的自由基[50]。
外源刺激秀丽隐杆线虫达到亚致死或全致死状态时,摄入具有抗氧化能力的活性因子可以减少体内的氧化损伤。Wang Erjia等[51]将秀丽隐杆线虫转移到含有200 μmol/L胡桃醌的平板上,用不同浓度的叶绿素进行处理,每1~2 h计录死亡线虫的个数。对照组的秀丽隐杆线虫在氧化应激9 h后死亡,低浓度的叶绿素可以显著减小胡桃醌诱导的急性氧化应激造成的损伤,延长秀丽隐杆线虫氧化应激后的存活时间,同时使sod-3表达水平提升;但是敲除daf-16的秀丽隐杆线虫寿命未延长,结果表明叶绿素的抗氧化活性可能取决于daf-16。Amigoni等[52]研究了绿色咖啡提取物(green coffe extract,GCE)对秀丽隐杆线虫的影响,将不同浓度的GCE处理的秀丽隐杆线虫暴露于10 mmol/L百草枯,与对照组相比,添加各浓度的GCE均延长了秀丽隐杆线虫的存活时间。添加1 000 mg/L GCE的秀丽隐杆线虫平均寿命显著延长了46.1%,具有一定的抗氧化应激保护作用。Augusti等[53]用微囊藻毒素-LR(microcystin-LR,MIC-LR)对秀丽隐杆线虫进行应激反应,评估不同浓度的MIC-LR对秀丽隐杆线虫存活率的影响。他们从万寿菊花中提取类胡萝卜素、叶黄素,测试其对MIC-LR毒性的保护作用。结果表明,类胡萝卜素、叶黄素阻止了秀丽隐杆线虫体内ROS的产生和MIC-LR诱导的氧化应激。Kim等[54]使用H2O2作为氧化应激诱导剂,在秀丽隐杆线虫体内检测雷公藤内脂醇的抗氧化活性。在氧化应激的条件下,与对照组相比,添加50 mg/L雷公藤内酯醇的秀丽隐杆线虫的存活率提升34.4%;测定雷公藤内酯醇处理组和对照组的荧光强度,发现雷公藤内脂醇治疗时间越长,对细胞内ROS水平影响越明显。雷公藤内酯在体内具有抗氧化应激的活性。王红等[55]用500 μmol/L胡桃醌作为氧化应激诱导剂,添加不同浓度紫薯提取物,发现秀丽隐杆线虫的平均寿命与最高寿命均高于对照组,同时其体内SOD和CAT活性均有所提高,脂褐素相对含量下降,表明紫薯提取物对胡桃醌氧化损伤线虫具有一定的保护作用。Won等[56]发现向秀丽隐杆线虫的食物中添加黄粉虫提取物,显著提高了秀丽隐杆线虫的氧化应激能力,同时提升了SOD的表达水平。Lee等[57]研究了巴西木素对秀丽隐杆线虫抗氧化应激的影响,在900 μmol/L胡桃醌诱导的氧化应激条件下,经巴西木素处理的秀丽隐杆线虫比对照组寿命更长,对照组的最长存活时间为12 h,添加50、100 μmol/L巴西木素的秀丽隐杆线虫最大存活时间分别延长至18、24 h。分光光度法测定发现巴西木素显著提升了SOD活力,同时使秀丽隐杆线虫体内的ROS水平降低10.4%。Seo等[58]研究了梓醇对秀丽隐杆线虫寿命的影响,结果表明添加25 μmol/L梓醇的秀丽隐杆线虫平均寿命与对照组相比提升了约28.5%,其体内的SOD和CAT活力分别增加了50.1%和46.4%。同时,秀丽隐杆线虫细胞内ROS水平降低了44.57%。Kim等[59]研究了三亚乌草对秀丽隐杆线虫体内SOD和CAT活力的影响,添加250 μg/mL三亚乌草提取物使SOD和CAT活力分别增加了约61.5%和211.8%。Ma Xiaoli等[60]给秀丽隐杆线虫喂食芝麻蛋白肽,与对照组相比,实验组秀丽隐杆线虫运动能力增强,体内的ROS水平降低,其可能是通过调节skn-1提升了秀丽隐杆线虫的抗氧化应激的能力。Srivastava等[61]发现水飞蓟素作用于秀丽隐杆线虫具有正向调节寿命、减少氧化应激的功效,喂食水飞蓟素的秀丽隐杆线虫ROS水平降低50.33%,sod-3表达水平显著提升,daf-16和skn-1表达上调。skn-1基因的表达会提升秀丽隐杆线虫抗氧化应激的能力[62]。如图2所示,秀丽隐杆线虫经过外源刺激后,可以通过daf-16激活sod-3降低细胞内ROS的水平,并通过skn-1表达延长应激损伤后的机体寿命。
3.3 脂肪代谢研究
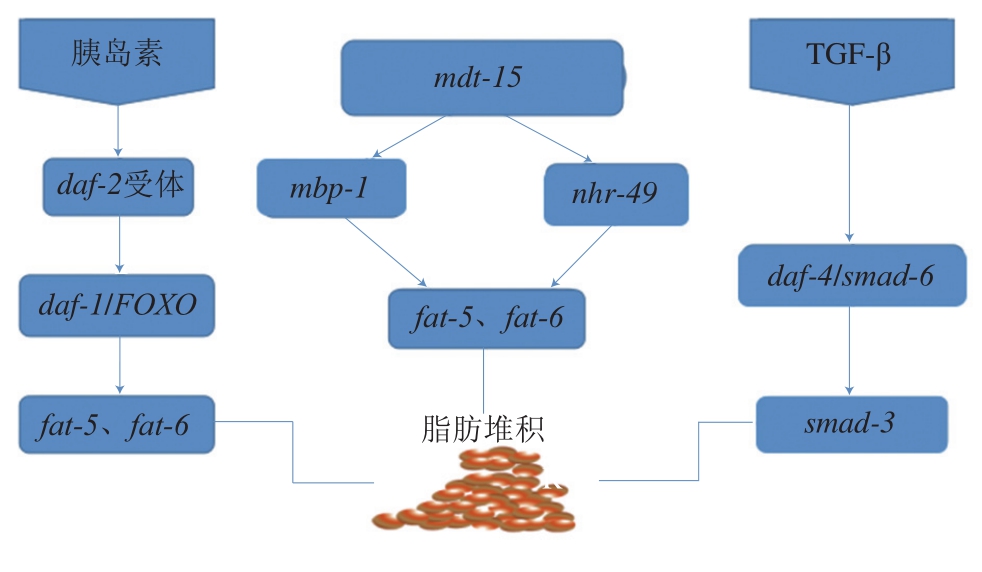
目前所知的具有降脂功能的食物主要成分可以分类为黄酮类、膳食纤维类、多糖类和多不饱和脂肪酸类[63]。秀丽隐杆线虫肠道通体透明、易于观察,且与人类肠道具有类似的功能,通过尼罗红和油红O染色可以了解脂肪细胞堆积的情况[64]。秀丽隐杆线虫的脂肪代谢通路主要为sbp-1/mdt-15信号通路、胰岛素信号通路、TGF-β信号通路、核激素受体调节信号通路[65]。影响秀丽隐杆线虫体内脂肪堆积的途径主要包括sbp-1/mdt-15表达增加[66]、daf-2激活[67]、TGF-β突变体导致糖原产生[68]、nhr-49基因受损和秀丽隐杆线虫体内脂肪堆积增加[69](图3)。
Shen Peiyi等[70]向秀丽隐杆线虫喂食白皮杉醇,然后进行尼罗红染色实验,发现白皮杉醇可以有效降低秀丽隐杆线虫体内脂肪堆积。向秀丽隐杆线虫喂食葡萄糖来增加脂肪堆积并建立肥胖模型,添加50、100 μmol/L白皮杉醇的秀丽隐杆线虫脂肪堆积分别下降了22%和44%。50、100 μmol/L白皮杉醇显著增加了hosl-1的表达,同时抑制了3T3-L1脂肪细胞的形成。Sun Quancai等[71]将蔓越莓提取物溶解于液体培养基中,发现0.16~2.00 mg/mL蔓越莓提取物可以有效降低野生型秀丽隐杆线虫中的三酰甘油酯含量,抑制3T3-L1脂肪细胞的形成。李萍等[72]将L1期秀丽隐杆线虫暴露于不同浓度姜黄素48 h后,用油红O对其肠道脂肪进行染色,结果表明与二甲基亚砜对照组相比,25 μmol/L以上姜黄素能够显著降低线虫肠道脂肪含量。Shen Peiyi等[73]研究了共轭亚油酸对秀丽隐杆线虫脂肪累积的影响,发现添加100 μmol/L共轭亚油酸纳米乳液组与对照组相比脂肪积累减少了29%。Gao Chenfei等[74]发现大麦膳食纤维可以抑制秀丽隐杆线虫体内的脂肪堆积,采用质量分数2%葡萄糖喂食秀丽隐杆线虫后进行尼罗红染色分析,与喂食大麦膳食纤维的秀丽隐杆线虫相比,其荧光强度减少19%~44%。王丽萍等[75]向秀丽隐杆线虫喂食葡萄糖建立脂肪堆积模型,通过油红O染色发现芦荟提取物具有一定的降脂作用。Nie Yu等[76]研究原花青素三聚体没食子酸酯对秀丽隐杆线虫脂质调节的作用,发现其可以有效降低甘油三酯水平。Kobayashi等[77]发现红茶多酚可以抑制胰脂肪酶活性,从而减少三酰甘油的吸收,有效降低甘油三酯含量。Jung等[78]同样发现绿茶提取物具有降低甘油三酯和非游离脂肪酸的能力。由于秀丽隐杆线虫自身不能合成胆固醇,对其在降脂方面的研究相对于小白鼠而言较少,但是各项研究均表明秀丽隐杆线虫脂肪代谢信号通路明确,且与人类高度相似,或许可以成为研究脂肪代谢的热门模式生物。
3.4 益生菌功能研究
大量研究表明,益生菌具有维持肠道内菌群平衡、调节宿主免疫、抑制致病菌的作用,成为近年来研究热点。周梦舟[79]以秀丽隐杆线虫为模型筛选抑制产肠毒的益生菌,利用产肠毒大肠杆菌(ETEC JG280)感染秀丽隐杆线虫,观察所筛选的乳酸菌对感染ETEC JG280的秀丽隐杆线虫的保护作用,结果表明乳酸菌对秀丽隐杆线虫具有保护作用,MAPK信号通路的pmk-1、daf-16基因表达上调。Li Ming等[80]研究表明嗜酸乳杆菌NCFM对感染粪肠球菌的秀丽隐杆线虫具有一定的保护作用,可以有效提高被感染后线虫的存活率。秀丽隐杆线虫食用嗜酸乳杆菌NCFM后,5 个宿主防御基因asp-10、clec-60、cpr-1、cpr-5和lys-5表达均显著上调。Kamaladevi等[81]使用肺炎克雷伯氏菌感染秀丽隐杆线虫后,采用13 株乳酸菌对感染的秀丽隐杆线虫进行保护,其中干酪乳杆菌显著提高了其存活率并减少了致病菌的定植,同时经过干酪乳杆菌处理的秀丽隐杆线虫pmk-1表达显著上调。Kamaladevi等[82]还发现干酪乳杆菌可以抑制秀丽隐杆线虫中马拉硫磷诱导的生理损伤,用干酪乳杆菌喂食暴露于马拉硫磷诱导的秀丽隐杆线虫可以保护其繁殖活性;对照组喂食大肠杆菌(OP50),其暴露于马拉硫磷的存活时间约为3 d,经干酪乳杆菌预处理后,其存活时间约为14 d左右,减少了秀丽隐杆线虫的头部痉挛和身体弯曲以及马拉硫磷造成的机体损伤,同时使mtl-1、mtl-2基因表达上调。Zhao Liang等[83]研究乳酸菌对秀丽隐杆线虫寿命的影响,发现秀丽隐杆线虫在喂食长双歧杆菌BB68后寿命延长了28%,长寿效应在其daf-16突变体中消失,同时通过免疫荧光显微镜发现tir-1和jnk-1参与daf-16磷酸化,因此,长双歧杆菌BB68可能是通过daf-16延长秀丽隐杆线虫寿命。Zhao Yunli等[84]发现乳酸菌可以保护石墨烯对秀丽隐杆线虫的毒性,秀丽隐杆线虫接触100 mg/L石墨烯会诱导肠内自由基的产生,喂食乳酸菌后得到明显抑制;同时乳酸菌可以减少秀丽隐杆线虫的身体弯曲。暴露于石墨烯的秀丽隐杆线虫acs-22表达显著下调,但喂食乳酸菌后暴露于石墨烯的秀丽隐杆线虫维持acs-22的正常表达;因此,乳酸菌维持肠道通透性可能取决于acs-22。Lee等[85]发现用热灭活的乳杆菌喂食秀丽隐杆线虫可以减轻革兰氏阴性菌的感染并延长秀丽隐杆线虫的寿命。乳酸菌具有抗炎作用[86],可以在人类肠道内定植[87],具有耐低pH值、耐胆盐、耐胃蛋白酶的能力[88],益生菌菌株通过调节p38/MAPK信号通路增强宿主免疫力,并可能通过同源途径影响秀丽隐杆线虫的免疫和衰老。如图4所示,乳酸菌可能通过调节daf-16基因表达延长秀丽隐杆线虫的寿命。
4 结 语
食品功能营养逐渐成为关注热点,对食品营养评价的研究日趋增多。将秀丽隐杆线虫作为载体,使食品营养活性因子与功能评价相结合,可以对食品活性因子的功能进行评价。秀丽隐杆线虫作为模式生物,其信号通路的研究相对比较透彻,与哺乳动物和人体的信号通路高度保守且具有同源性,具有成本低、高效能的优点。但也存在肠道构造同人类相比相对简单、体内仅存在先天免疫应答等问题。然而由于食品营养活性成分体内模型筛选耗时较长,而秀丽隐杆线虫生长周期较短;因此,秀丽隐杆线虫是食品营养活性成分体内模型筛选的热门模式生物,极大提高了食品营养活性成分体内模型筛选的效率。目前,对秀丽隐杆线虫抗衰老和抗氧化应激的研究比较成熟,其脂肪代谢研究相对前两者而言比较薄弱,益生菌研究比较单一。选择评价周期较短的秀丽隐杆线虫对具有降脂功能的活性因子和具有活性功能的益生菌筛选势在必行,对于降脂类和活性功能益生菌食品的研发十分必要。利用秀丽隐杆线虫对食品营养进行评价,不但可以避免机体损伤,同时可以验证活性成分的营养功能,为代谢障碍疾病和氧化应激损伤提供参考,也为功能性食品的研发提供模式生物。
参考文献:
[1] BRENNER S. The genetics of Caenorhabditis elegans[J]. Genetics,1974, 77(1): 71-94.
[2] VIGNESHKUMAR B, PANDIAN S K, BALAMURUGAN K.Catalase activity and innate immune response of Caenorhabditis elegans against the heavy metal toxin lead[J]. Environmental Toxicology, 2013, 28(6): 313-321. DOI:10.1002/tox.20722.
[3] WAN H, LI D. Highly efficient biotransformation of ginsenoside Rb1 and Rg3 using β-galactosidase from Aspergillus sp[J]. RSC Advances,2015, 96: 78874-78879. DOI:10.1039/C5RA11519A.
[4] KWON G, LEE J, KOH J H, et al. Lifespan Extension of Caenorhabditis elegans by Butyricicoccus pullicaecorum and Megasphaera elsdenii with probiotic potential[J]. Current Microbiology,2018, 75(5): 557-564. DOI:10.1007/s00284-017-1416-6.
[5] PINCUS Z, MAZER T C, SLACK F J. Autofluorescence as a measure of senescence in C. elegans: look to red, not blue or green[J]. Aging,2016, 8(5): 889-898. DOI:10.18632/aging.100936.
[6] LAI C H, CHOU C Y, CH’ANG L Y, et al. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics[J]. Genome Research, 2000, 10(5): 703-713.DOI:10.1101/gr.10.5.703.
[7] HARRIS T W, CHEN N, CUNNINGHAM F, et al. WormBase: a multi-species resource for nematode biology and genomics[J]. Nucleic Acids Research, 2004, 32: 411-417. DOI:10.1093/nar/gkh066.
[8] KUDRON M M, VICTORSEN A, GEVIRTZMAN L, et al. The modERN resource: genome-wide binding profiles for hundreds of Drosophila and Caenorhabditis elegans transcription factors[J].Genetics, 2018, 208(3): 937-949. DOI:10.1534/genetics.117.300657.
[9] SRINIVASAN S. Regulation of body fat in Caenorhabditis elegans[J].Annual Review of Physiology, 2014, 77(1): 161-178. DOI:10.1146/annurev-physiol-021014-071704.
[10] NOBLE T, STIEGLITZ J, SRINIVASAN S. An integrated serotonin and octopamine neuronal circuit directs the release of an endocrine signal to control C. elegans body fat[J]. Cell Metabolism, 2013, 18(5):672-684. DOI:10.1016/j.cmet.2013.09.007.
[11] KWON E S, NARASIMHAN S D, YEN K, et al. A new DAF-16 isoform regulates longevity[J]. Nature, 2010, 466: 498-502.DOI:10.1038/nature09184.
[12] KENYON C. The first long-lived mutants: discovery of the insulin/IGF-1 pathway for ageing[J]. Philosophical Transactions of the Royal Society B, 2011, 366: 9-16. DOI:10.1098/rstb.2010.0276.
[13] BOLZ D D, TENOR J L, ABALLAY A. A conserved PMK-1/p38 MAPK is required in caenorhabditis elegans tissue-specific immune response to Yersinia pestis infection[J]. Journal of Biological Chemistry, 2010, 285(14): 10832-10840. DOI:10.1074/jbc.M109.091629.
[14] SHIVERS R P, YOUNGMAN M J, KIM D H. Transcriptional responses to pathogens in Caenorhabditis elegans[J]. Current Opinion in Microbiology, 2008, 11(3): 251-256. DOI:10.1016/j.mib.2008.05.014.
[15] KENYON C, CHANG J, GENSCH E, et al. A C. elegans, mutant that lives twice as long as wild type[J]. Nature, 1993, 366: 461-464.DOI:10.1038/366461a0.
[16] SHEN C Y, JIANG J G, LI Y, et al. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional chinese medicine:pharmacological mechanisms and implications for drug discovery[J].British Journal of Pharmacology, 2016, 174(11): 1395-1425.DOI:10.1111/bph.13631.
[17] BALLA K M, TROEMEL E R. Caenorhabditis elegans as a model for intracellular pathogen infection[J]. Cellular Microbiology, 2013, 15(8):1313-1322. DOI:10.1111/cmi.12152.
[18] PARADIS S, RUVKUN G. Caenorhabditis elegans akt/pkb transduces insulin receptor-like signals from age-1 PI3 kinase to the daf-16 transcription factor[J]. Genes Development, 1998, 12(16): 2488-2498.DOI:10.1101/gad.12.16.2488.
[19] HARUKA H, UNO M, HONJOH S, et al. Octopamine enhances oxidative stress resistance through the fasting-responsive transcription factor DAF-16/FOXO in C. elegans[J]. Genes to Cells, 2017, 22(2):210-219. DOI:10.1111/gtc.12469.
[20] MUKHOPADHYAY A, OH S W, TISSENBAUM H A. Worming pathways to and from DAF-16/FOXO[J]. Experimental Gerontology,2006, 41(10): 928-934. DOI:10.1016/j.exger.2006.05.020.
[21] HONDA Y, TANAKA M, HONDA S. Redox regulation, gene expression and longevity[J]. Geriatrics & Gerontology International,2010, 10(Suppl 1): S59-S69. DOI:10.1111/j.1447-0594.2010.00591.x.
[22] CHI W, CHEN S, HIGASHIBATA A, et al. Changes of muscle-related genes and proteins after spaceflight in Caenorhabditis elegans[J].Progress in Biochemistry & Biophysics, 2008, 35(10): 1195-1201.DOI:10.1016/S1001-8042(08)60040-8.
[23] O’NEILL C, KIELY A P, COAKLEY M F, et al. Insulin and IGF-1 signalling: longevity, protein homoeostasis and Alzheimer’s disease[J]. Biochemical Society Transactions, 2012, 40(4): 721-727.DOI:10.1042/BST20120080.
[24] BAI M, VOZDEK R, HNÍZDA A, et al. Conserved roles of C. elegans and human MANFs in sulfatide binding and cytoprotection[J]. Nature Communications, 2018, 9(1): 897-908. DOI:10.1038/s41467-018-03355-0.
[25] KYRIAKIS J M, AVRUCH J. Protein kinase cascades activated by stress and inflammatory cytokines[J]. Bioessays, 1996, 18(7): 567-577. DOI:10.1002/bies.950180708.
[26] CHANG L, KARIN M. Mammalian MAP kinase signalling cascades[J]. Nature, 2001, 410: 37-40. DOI:10.1038/35065000.
[27] KASSAHUN H, SENGUPTA T, SCHIAVI A, et al. Constitutive MAP-kinase activation suppresses germline apoptosis in NTH-1 DNA glycosylase deficient C. elegans[J]. DNA Repair, 2018, 61: 46-55.DOI:10.1016/j.dnarep.2017.11.009.
[28] MORITA K, FLEMMING A J, SUGIHARA Y, et al. A Caenorhabditis elegans TGF-β, DBL-1, controls the expression of LON-1, a PR-related protein, that regulates polyploidization and body length[J]. The eMBO Journal, 2002, 21(5): 1063-1073. DOI:10.1093/emboj/21.5.1063.
[29] NAKA K, HOSHII T, MURAGUCHI T, et al. TGF-β-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia[J]. Nature, 2010, 463: 676-680. DOI:10.1038/nature08734.
[30] ROBERTS A F, GUMIENNY T L, GLEASON R J, et al. Regulation of genes affecting body size and innate immunity by the DBL-1/BMP-like pathway in Caenorhabditis elegans[J]. BMC Developmental Biology, 2010, 10(1): 1-10. DOI:10.1186/1471-213X-10-61.
[31] ZUGASTI O, EWBANK J J. Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-β signaling pathway in Caenorhabditis elegans epidermis[J]. Nature Immunology,2009, 10(3): 249-256. DOI:10.1038/ni.1700.
[32] HE K, ZHOU T, SHAO J, et al. Dynamic regulation of genetic pathways and targets during aging in Caenorhabditis elegans[J].Aging, 2014, 6(3): 215-230. DOI:10.18632/aging.100648.
[33] NEWELL B S L, CYPSER J R, KECHRIS K, et al. Movement decline across lifespan of Caenorhabditis elegans mutants in the insulin/insulin-like signaling pathway[J]. Aging Cell, 2017, 17(1): e12704.DOI:10.1111/acel.12704.
[34] MERGOUD A D L, MOLIN L, PIERSON L, et al. UNC-120/SRF independently controls muscle aging and lifespan in Caenorhabditis elegans[J]. Aging Cell, 2018, 17(2): e12713. DOI:10.1111/acel.12713.
[35] KIM Y S, SEO H W, LEE M H, et al. Protocatechuic acid extends lifespan and increases stress resistance in Caenorhabditis elegans[J].Archives of Pharmacal Research, 2014, 37(2): 245-252. DOI:10.1007/s12272-013-0183-6.
[36] WOOD J G, ROGINA B, LAVU S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans[J]. Nature, 2004,430: 686-689. DOI:10.1038/nature02789.
[37] CHEN Wei, LIN Hongru, WEI Congmin, et al. Echinacoside, a phenylethanoid glycoside from Cistanche deserticola, extends lifespan of Caenorhabditis elegans, and protects from a β-inducedtoxicity[J].Biogerontology, 2017(3): 1-19. DOI:10.1007/s10522-017-9738-0.
[38] FEI Tianyi, FEI Jian, HUANG Fang, et al. The anti-aging and antioxidation effects of tea water extract in Caenorhabditis elegans[J].Experimental Gerontology, 2017, 97: 89-96. DOI:10.1016/j.exger.2017.07.015 .
[39] TAIRA N, NGUYEN B C Q, TU P T B, et al. Effect of okinawa propolis on PAK1 activity, Caenorhabditis elegans longevity,melanogenesis, and growth of cancer cells[J]. Journal of Agricultural and Food Chemistry, 2016, 64(27): 5484-5489. DOI:10.1021/acs.jafc.6b01785.
[40] ZHENG Shanqing, HUANG Xiaobing, XING Tikun, et al.Chlorogenic acid extends the lifespan of Caenorhabditis elegans via insulin/IGF-1 signaling pathway[J]. Journal of Gerontology Series A-biological Sciences and Medical Sciences, 2017, 72(4): 464-472.DOI:10.1093/gerona/glw105.
[41] 刘冰冰. 铁皮石斛多糖对秀丽隐杆线虫寿命的影响及其机制的研究[D]. 北京: 北京林业大学, 2016: 2-11.
[42] MENG Fanhui, LI Jun, RAO Yanqiu, et al. Gengnianchun extends the lifespan of Caenorhabditis elegans via the insulin/IGF-1 signalling pathway[J]. Oxidative Medicine & Cellular Longevity, 2018(6): 1-10.DOI:10.1155/2018/4740739.
[43] LIAO V H C, YU C W, CHU Y J, et al. Curcumin-mediated lifespan extension in Caenorhabditis elegans[J]. Mechanisms of Ageing & Development, 2011, 132(10): 480-487. DOI:10.1016/j.mad.2011.07.008.
[44] HAVERMANN S, HUMPF H U, WÄTJEN W. Baicalein modulates stress-resistance and life span in Caenorhabditis elegans, via SKN-1 but not DAF-16[J]. Fitoterapia, 2016, 113(6): 123-127. DOI:10.1016/j.fitote.2016.06.018.
[45] DEHGHAN E, ZHANG Y, SAREMI B, et al. Hydralazine induces stress resistance and extends C. elegans lifespan by activating the NRF2/SKN-1 signalling pathway[J]. Nature Communications, 2017,8(1): 2223-2231. DOI:10.1038/s41467-017-02394-3.
[46] KÖHNLEIN K, URBAN N, STEINBRENNER H, et al. P172-two putative selenium binding proteins as modulators of C. elegans, stress response and lifespan[J]. Free Radical Biology and Medicine, 2017,108: 77-85. DOI:10.1016/j.freeradbiomed.2017.04.257.
[47] 李芳. 白藜芦醇对环境因子致氧化损伤干预作用的生化分析[D]. 长沙: 湖南大学, 2013: 1. DOI:10.7666/d.Y2358380.
[48] FENG S, CHENG H, XU Z, et al. Thermal stress resistance and aging effects of Panax notoginseng polysaccharides on Caenorhabditis elegans[J]. International Journal of Biological Macromolecules, 2015,81(7): 188-194. DOI:10.1016/j.ijbiomac.2015.07.057.
[49] KADLECOVÃ A, JIRSA T, NOVÃ K O, et al. Natural plant hormones cytokinins increase stress resistance and longevity of Caenorhabditis elegans[J]. Biogerontology, 2017, 19(1): 1-12. DOI:10.1007/s10522-017-9742-4.
[50] ESCOBEDO J, PUCCI A M, KOH T J. HSP25 protects skeletal muscle cells against oxidative stress[J]. Free Radical Biology & Medicine,2004, 37(9): 1455-1462. DOI:10.1016/j.freeradbiomed.2004.07.024.
[51] WANG Erjia, MICHAEL W. Chlorophyll enhances oxidative stress tolerance in Caenorhabditis elegans and extends its lifespan[J]. PeerJ,2016, 4(3): e1879. DOI:10.7717/peerj.1879.
[52] AMIGONI L, STUKNYTĖ M, CIARAMELLI C, et al. Green coffee extract enhances oxidative stress resistance and delays aging in Caenorhabditis elegans[J]. Journal of Functional Foods, 2017, 33(10):297-306. DOI:10.1016/j.jff.2017.03.056.
[53] AUGUSTI P R, BRASIL A V S, SOUTO C, et al. Microcystin-LR exposure induces oxidative damage in Caenorhabditis elegans:protective effect of lutein extracted from marigold flowers[J]. Food and Chemical Toxicology, 2017, 109(Pt1): 60-67. DOI:10.1016/j.fct.2017.08.045.
[54] KIM S J, BEAK S, PARK S. Supplementation with triptolide increases resistance to environmental stressors and lifespan in C. elegans[J]. Journal of Food Science, 2017, 82(6): 1484-1490. DOI:10.1111/1750-3841.13720.
[55] 王红, 张晓寒, 程静, 等. 紫薯提取物对秀丽隐杆线虫抗氧化作用的影响[J]. 食品科学, 2017, 38(23): 165-170. DOI:10.7506/spkx1002-6630-201723026.
[56] WON S M, CHA H U, YI S S, et al. Tenebrio molitor extracts modulate the response to environmental stressors and extend lifespan in Caenorhabditis elegans[J]. Journal of Medicinal Food, 2016,19(10): 938-944. DOI:10.1089/jmf.2016.3729.
[57] LEE E B, MING M X, KIM D K. Lifespan-extending and stress resistance properties of brazilin from Caesalpinia sappan, in Caenorhabditis elegans[J]. Archives of Pharmacal Research, 2017,40(7): 825-835. DOI:10.1007/s12272-017-0920-3.
[58] SEO H W, CHEON S M, LEE M H, et al. Catalpol modulates lifespan via DAF-16/FOXO and SKN-1/Nrf2 activation in Caenorhabditis elegans[J]. Evidence-Based Complementary and Alternative Medicine,2015, 2015: 524878. DOI:10.1155/2015/524878.
[59] KIM H N, SEO H W, KIM B S, et al. Lindera obtusiloba extends lifespan of Caenorhabditis elegans[J]. Natural Product Sciences, 2015,21(2): 128-133.
[60] MA Xiaoli, CUI Xiaodong, LI Jiao, et al. Peptides from sesame cake reduce oxidative stress and amyloid-β-induced toxicity by upregulation of SKN-1 in a transgenic Caenorhabditis elegans model of Alzheimer’s disease[J]. Journal of Functional Foods, 2017, 12(39):287-298. DOI:10.1016/j.jff.2017.10.032.
[61] SRIVASTAVA S, SAMMI S R, LAXMAN T S, et al. Silymarin promotes longevity and alleviates Parkinson’s associated pathologies in Caenorhabditis elegans[J]. Journal of Functional Foods, 2017, 31:32-43. DOI:10.1016/j.jff.2017.01.029.
[62] TULLET J M A, GREEN J W, AU C, et al. The SKN-1/Nrf2 transcription factor can protect against oxidative stress and increase lifespan in C. elegans by distinct mechanisms[J]. Aging Cell, 2017,16(5): 1191-1194. DOI:10.1111/acel.12627.
[63] JANG J, JUNG Y, CHAE S, et al. Gangjihwan, a polyherbal composition, inhibits fat accumulation through the modulation of lipogenic transcription factors SREBP1C, PPARγ and C/EBPα[J].Journal of Ethnopharmacology, 2017, 210: 10-22. DOI:10.1016/j.jep.2017.08.024.
[64] LEE H, KIM J, PARK J Y, et al. Processed Panax ginseng, sun ginseng,inhibits the differentiation and proliferation of 3T3-L1 preadipocytes and fat accumulation in Caenorhabditis elegans[J]. Journal of Ginseng Research, 2016, 41(3): 257-267. DOI:10.1016/j.jgr.2016.04.004.
[65] SHEN P, YUE Y, PARK Y. A living model for obesity and aging research: Caenorhabditis elegans[J]. Critical Reviews in Food Science &Nutrition, 2016(5): 741-754. DOI:10.1080/10408398.2016.1220914.
[66] ASHRAFI K, CHANG F Y, WATTS J L, et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes[J]. Nature,2003, 421: 268-272. DOI:10.1038/nature01279.
[67] BROCK T J, BROWSE J, WATTS J L. Fatty acid desaturation and the regulation of adiposity in Caenorhabditis elegans[J]. Genetics, 2007,176(2): 865-875. DOI:10.1534/genetics.107.071860.
[68] PARK S, PAIK Y K. Genetic deficiency in neuronal peroxisomal fatty acid β-oxidation causes the interruption of dauer development in Caenorhabditis elegans[J]. Scientific Reports, 2017, 7(1): 9358.DOI:10.1038/s41598-017-10020-x.
[69] MORENO-ARRIOLA E, EL HAFIDI M, ORTEGA-CUÉLLAR D,et al. AMP-activated protein kinase regulates oxidative metabolism in Caenorhabditis elegans through the NHR-49 and MDT-15 transcriptional regulators[J]. PLoS ONE, 2017, 11(1): 1-10.DOI:10.1371/journal.pone.0148089.
[70] SHEN Peiyi, YUE Yiren, KIM K H, et al. Piceatannol reduces fat accumulation in Caenorhabditis elegans[J]. Journal of Medicinal Food, 2017, 20(9): 887-894. DOI:10.1089/jmf.2016.0179.
[71] SUN Quancai, YUE Yiren, SHEN Peiyi, et al. Cranberry product decreases fat accumulation in Caenorhabditis elegans[J]. Journal of Medicinal Food, 2016, 19(4): 427-433. DOI:10.1089/jmf.2015.0133.
[72] 李萍, 米生权, 冯亚芳, 等. 姜黄素对秀丽隐杆线虫降脂及抗氧化保护作用[J]. 食品工业科技, 2017, 38(14): 289-293. DOI:10.13386/j.issn1002-0306.2017.14.057.
[73] SHEN Peiyi, KERSHAW J C, YUE Yiren, et al. Effects of conjugated linoleic acid (CLA) on fat accumulation, activity, and proteomics analysis in Caenorhabditis elegans[J]. Food Chemistry, 2018, 249:193-201. DOI:10.1016/j.foodchem.2018.01.017.
[74] GAO Chenfei, KING M L, FITZPATRICK Z L, et al. Prowashonupana,barley dietary fibre reduces body fat and increases insulin sensitivity in Caenorhabditis elegans, model[J]. Journal of Functional Foods, 2015,18: 564-574. DOI:10.1016/j.jff.2015.08.014.
[75] 王丽萍, 徐佳, 王琪菲, 等. 以线虫为模型考察中国芦荟提取物的降脂作用[J]. 吉林大学学报(理学版), 2016, 54(5): 1181-1185.DOI:10.13413/j.cnki.jdxblxb.2016.05.42.
[76] NIE Yu, LITTLETON B, KAVANAGH T, et al. Proanthocyanidin trimer gallate modulates lipid deposition and fatty acid desaturation in Caenorhabditis elegans[J]. FASEB Journal Official Publication of the Federation of American Societies for Experimental Biology, 2017,31(11): 4891-4902. DOI:10.1096/fj.201700438R.
[77] KOBAYASHI M, ICHITANI M, SUZUKI Y, et al. Black-tea polyphenols suppress postprandial hypertriacylglycerolemia by suppressing lymphatic transport of dietary fat in rats[J]. Journal of Agricultural and Food Chemistry, 2009, 57(15): 7131-7136.DOI:10.1021/jf900855v.
[78] JUNG M H, SEONG P N, KIM M H, et al. Effect of green tea extract microencapsulation on hypertriglyceridemia and cardiovascular tissues in high fructose-fed rats[J]. Nutrition Research & Practice, 2013, 7(5):366-372. DOI:10.4162/nrp.2013.7.5.366.
[79] 周梦舟. 以秀丽隐杆线虫筛选抑制产肠毒大肠杆菌的益生菌及其作用机理[D]. 无锡: 江南大学, 2014: 12-20.
[80] LI Ming, LEE K, MIN H, et al. Lactobacillus-derived extracellular vesicles enhance host immune responses against vancomycin-resistant enterococci[J]. BMC Microbiology, 2017, 17(1): 66-72. DOI:10.1186/s12866-017-0977-7.
[81] KAMALADEVI A, BALAMURUGAN K. Lactobacillus casei triggers a TLR mediated RACK-1 dependent p38 MAPK pathway in Caenorhabditis elegans to resist Klebsiella pneumoniae infection[J].Food & Function, 2016, 7(7): 3211-3223. DOI:10.1039/C6FO00510A.
[82] KAMALADEVI A, GANGULI A, BALAMURUGAN K.Lactobacillus casei stimulates phase-II detoxification system and rescues malathion-induced physiological impairments in Caenorhabditis elegans[J]. Comparative Biochemistry & Physiology Part C Toxicology & Pharmacology, 2015, 179: 19-28. DOI:10.1016/j.cbpc.2015.08.004.
[83] ZHAO Liang, ZHAO Yang, LIU Ruihai, et al. The transcription factor DAF-16 is essential for increased longevity in C. elegans exposed to Bifidobacterium longum BB68[J]. Scientific Reports, 2017, 7(1): 7408.DOI:10.1038/s41598-017-07974-3.
[84] ZHAO Yunli, YU Xiaoming, JIA Ruhua, et al. Lactic acid bacteria protects Caenorhabditis elegans from toxicity of graphene oxide by maintaining normal intestinal permeability under different genetic backgrounds[J]. Scientific Reports, 2015, 5: 17233. DOI:10.1038/srep17233.
[85] LEE J, CHOE J, KIM J, et al. Heat-killed Lactobacillus spp. cells enhance survivals of Caenorhabditis elegans against Salmonella and Yersinia infections[J]. Letters in Applied Microbiology, 2016, 61(6):523-530. DOI:10.1111/lam.12478.
[86] ZANNI E, SCHIFANO E, MOTTA S, et al. Combination of metabolomic and proteomic analysis revealed different features among Lactobacillus delbrueckii subspecies bulgaricus and lactis strains while in vivo testing in the model organism Caenorhabditis elegans highlighted probiotic propertie[J]. Frontiers in Microbiology, 2017, 8:1206. DOI:10.3389/fmicb.2017.01206.
[87] MARTORELL P, LLOPIS S, GONZÁLEZ N, et al. Probiotic strain Bifidobacterium animalis subsp. lactis CECT 8145 reduces fat content and modulates lipid metabolism and antioxidant response in Caenorhabditis elegans[J]. Journal of Agricultural and Food Chemistry, 2016, 64(17): 3462-3472. DOI:10.1021/acs.jafc.5b05934.
[88] JANG S Y, HEO J, PARK M R, et al. Genome characteristics of Lactobacillus fermentum strain JDFM216 for application as probiotic bacteria[J]. Journal of Microbiology & Biotechnology, 2017, 27(7):1266-1271. DOI:10.4014/jmb.1703.03013.
Food Nutritional Evaluation: Caenorhabditis elegans as a Model Organism
YANG Fan1, XIA Chengcheng1, ZHONG Xiaoling1, LI Qin1, LI Xi1, ZHANG Zhiyuan2, SHI Wenbo3, XU Ning1, WU Qian1,HU Yong1, LIU Zhijie1, WANG Chao1, ZHOU Mengzhou1,*
(1. Hubei Cooperative Innovation Center for Industrial Fermentation, Research Center of Food Fermentation Engineering and Technology of Hubei, Hubei University of Technology, Wuhan 430068, China; 2. Wuhan Environmental Testing Center,Wuhan 430015, China; 3. Hubei Accurate Inspection Testing Co. Ltd., Wuhan 430077, China)
Abstract: Food nutrition is always a research focus and hotspot in the field of food science. The consumption of functional foods has increased year by year. Model organisms are essential in the evaluation of food functionalities. Caenorhabditis elegans, a small free-living soil nematode, has been extensively used as an experimental in vivo system for biological studies due to its small size, short generation time, and suitability for genetic analysis. In this article, we summarize and review the homology of signaling pathways between C. elegans and humans, the classification and evaluation of functional food factors, and recent progress in the application of C. elegans in food nutritional evaluation, with the aim of providing a basis for food nutrition research and the development of functional foods.
Keywords: food nutrition; Caenorhabditis elegans; signaling pathways; nutritional evaluation
收稿日期:2018-04-24
基金项目:国家自然科学基金青年科学基金项目(31601455);“十三五”国家重点研发计划重点专项(2016YFD0400701);湖北省粮食局科技创新项目(鄂财商发[2017]58号)
第一作者简介:杨番(1995—)(ORCID: 0000-0002-9642-702X),男,硕士研究生,研究方向为食品微生物。E-mail: 503485584@qq.com
*通信作者简介:周梦舟(1986—)(ORCID: 0000-0001-6317-3142),男,副教授,博士,研究方向为食品营养与安全、食品微生物。E-mail: zmzkelvin@163.com
DOI:10.7506/spkx1002-6630-20180424-317
中图分类号:TS201.4
文献标志码:A
文章编号:1002-6630(2019)11-0268-09
引文格式:
杨番, 夏程程, 钟晓凌, 等. 秀丽隐杆线虫模型在食品营养评价中的应用研究进展[J]. 食品科学, 2019, 40(11): 268-276.DOI:10.7506/spkx1002-6630-20180424-317. http://www.spkx.net.cn
YANG Fan, XIA Chengcheng, ZHONG Xiaoling, et al. Food nutritional evaluation: Caenorhabditis elegans as a model organism[J]. Food Science, 2019, 40(11): 268-276. (in Chinese with English abstract) DOI:10.7506/spkx1002-6630-20180424-317. http://www.spkx.net.cn