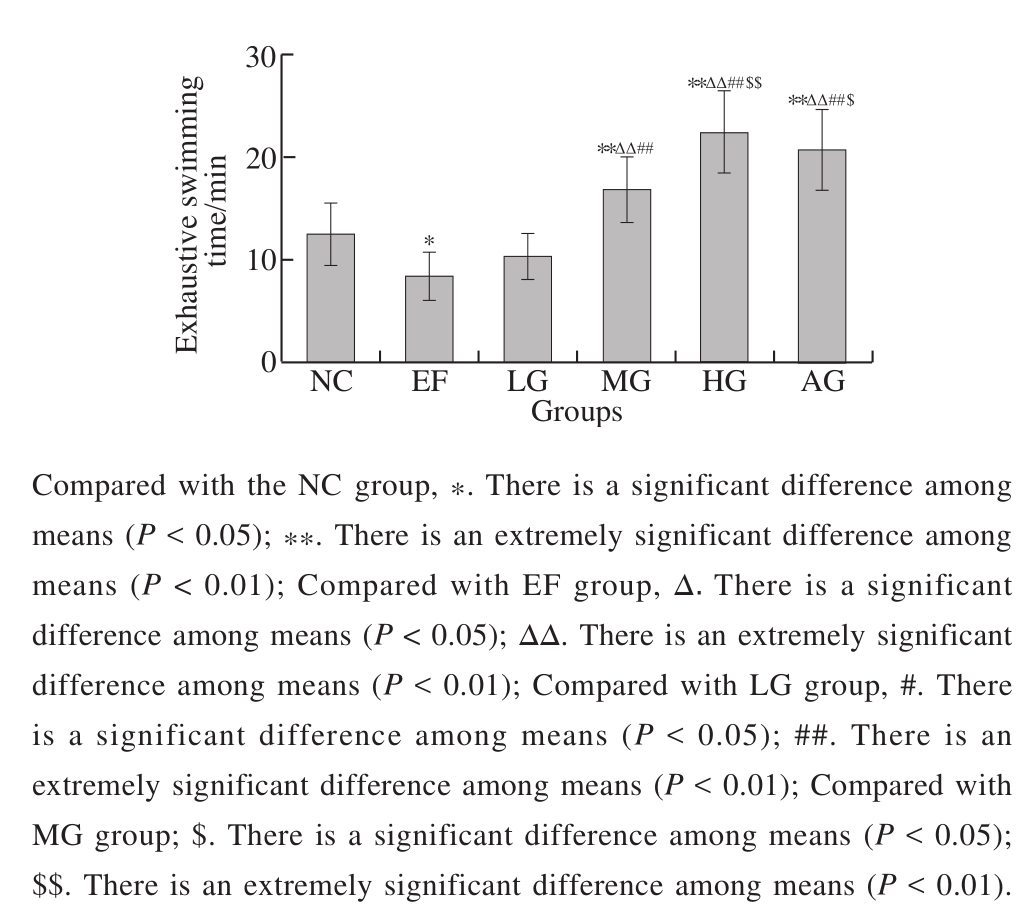
Fig. 1 Effect of GSH on exhaustive swimming time of mice
Glutathione (GSH) is a tripeptide composed of L-glutamic acid, L-cysteine and glycine. It is widely used in bioactive non protein thiol compounds in organisms. The chemical structure of GSH determines that its structure is very stable. GSH has a high biological activity. It plays an important role in antioxidant defense, protein folding and signal transduction. Furthermore, it also participates in the regulation of cell proliferation, apoptosis, immune function and liver fi brosis[1]. The depletion of GSH is associated with a variety of diseases, such as cancer, neurodegenerative and cardiovascular disease etc. in humans[2]. In addition,GSH can exclude toxic compounds in the body, such as acrylonitrile, fluoride and carbon monoxide, so as to play a role in neutralization and detoxification. It also has the functions of scavenging oxygen free radicals, improving microcirculation and inhibiting the production of various inflammatory factors[3]. In summary, GSH has a variety of important activities and attracts more and more interest from many pharmacologists. However, the anti-fatigue effect of GSH and its mechanism are rarely yet reported.
Fatigue refers to the syndrome caused by physical and/or mental fatigue, which is unable to continue to move at the same intensity and lead to deterioration. Because of its negative impact on the somatic and cognitive functions,fatigue has become a focused health problem[4]. Some studies indicated that fatigue is mainly due to energy depletion,including the final product of fatigue accumulation, the body’s internal environment disorder, blood glucose level and reduce the consumption of glycogen. Fatigue often occurred in aging, cancer, depression, human immunodeficiency virus infection, multiple sclerosis and Parkinson’s disease which might seriously affects the life quality[5]. However, there are few excellent clinical therapies or pharmacologic drugs for fatigue at present[6]. Therefore, it is very important to develop safe and effective anti-fatigue drugs.
Xu et al found that after exercise, supplementation of nutrients can control the proteins, carbohydrates and amino acids, eliminating the accumulation of harmful metabolites and repairing the body injury to promote fatigue recovery[7].Most protein hydrolysates and polypeptides (such as GSH)have the advantages of high bioactivity, low molecular mass, high absorbency and low toxicity. In addition, some researchers have also confirmed that some bioactive protein hydrolysates and peptides help combat and improve body fatigue. Therefore, it is a very sensible choice to develop GSH into anti-fatigue drugs[4]. The purpose of this study is to investigate the effects of GSH on the biochemical indexes and c-fos expression in brain tissue of exercise-induced fatigue mice. The results will provide a reference for the extensive utilization of GSH resources and the development of a new type of anti-fatigue supplement.
Male Kunming mice (216, massing 18-22 g) were used in this experiment and purchased from Qingdao Daren Fortune Animal Technology Co. Ltd. (Animal License No.: SCXK(Jing)2016-0002). The mice were housed at(22 ± 2)℃ and relative humidity 50%-60% with a 12 h:12 h light-dark cycle. Standard food and water are freely available. The Canadian Council on Animal Care guidelines was followed in all animal procedures in this experiment.The protocols for animal experiments were approved by the Animal Ethics Committee of Qingdao University of Science and Technology.
lactic acid (LAC), lactic dehydrogenase (LDH),creatine phosphokinase (CK), blood urea nitrogen (BUN),superoxide dismutase (SOD), malondialdehyde (MDA) level detection kits, and dopamine (DA), 5-hydroxytryptamine(5-HT) enzyme-linked immunosorbent assay (ELISA) kits Nanjing Jiancheng Bioengineering Institute; Reduced GSH(> 98% purity) Hubei Kangbaotai Fine-Chemicals Co.Ltd.; American ginseng (Panax quinquefolius) (AG) buccal tablets Xiamen Yongfuxing Health Products Co. Ltd.;Anthranone Sinopharm Chemical Reagent Co. Ltd.;c-fos antibody Abcam Trading (Shanghai) Co. Ltd.;Immunohistochemical Staining Kit and 3,3-diaminobenzidine(DAB) Staining Kit Bioss Antibodies Co., Ltd.;Hematoxylin staining solution Wuxi Jiangyuan Industrial Technology and Trade Co. Ltd.; All the other chemicals used were of analytical grade.
Glass swimming pool (40 cm × 55 cm × 55 cm) was self-made; FC type enzyme immunoassay analyzer Jinan Boxin Biotechnology Co. Ltd.; TD-24K centrifuge Xiangyi Centrifuge Co. Ltd.; BX50 microscope and DP50 digital camera Olympic Limbus Co. Ltd. (Japan).
1.3.1 Animals treatment
After 7-days acclimatization, the mice were randomly divided into 3 experimental sets. Each set of mice was divided into 6 groups (n = 12): normal control group (NC), exercise fatigue group (EF), low-dose GSH group (LG), medium-dose GSH group (MG) and high-dose GSH group (HG), positive control group (AG). All mice that were included in the experiment carried out adaptive swimming training 1 time per day and 20 min per time for 3 days. The water depth was 40 cm.The temperature was maintained at (30 ± 2) ℃ when mice were swimming. From the 4th day on, mice in LG, MG and HG groups were administrated with 0.4 mL of 0.2, 0.4, 0.8 g/kg GSH, respectively. Mice in AG group were given 0.6 g/kg of AG. The mice in NC and EF groups were administrated with the same volume of normal saline, respectively. The swimming training began at 30 min after administration on the 10th day for all mice except NC group. The training plan was as follows: From 1st day to the 7th day, the mice swam once for 2 h, while twice from 8th to 10th day with a 3 h interval. In the process of the swimming, once the movement coordination decreased significantly and sank repeatedly,the mice were removed and had a rest for 3 min, and then put into the pool to swim. Each time of swimming lasted at least for 2 h. The sticks were used to drive the mice to move when they stopped swimming. The mice were administrated for 10 days and then were treated for further experiments after the last administration. The mice in NC group were kept in the same condition while did not participate in swimming training.
1.3.2 Record of exhaustive swimming time
Mice in experimental set 1 were used to record exhaustive swimming time. Thirty min after the last administration on the 10th day, the mice were forced to swim with mass-loaded instead of swimming training. The procedure was described as follows: the mice were placed individually in a swimming pool with a tin wire (7% of body mass) loaded on the tail root. The swimming period was regarded as the time spent by the mice from floating in the water with struggling and making necessary movements to exhausting its strength. The exhaustive swimming time was recorded exactly when the strength of the mice was depleted and it could not rise to the surface for more than 8 s.
1.3.3 Biochemical analysis
Mice in experimental set 2 were used for biochemical analysis. One hour after the last swimming training on the 10th day, blood was withdrawn from the eyeballs to separate serum by centrifugation at 2 500 r/min, 4 ℃ for 15 min.Then the livers and hypothalamus (PVN) were dissected out and washed in ice-cold saline patted dry to prepare the 10% (m/m) homogenates by centrifugation at 4 000 r/min,4 ℃ for 15 min. Levels of serum LAC, LDH, CK, BUN,liver SOD activity and MDA level were measured by commercial detection kits following the recommended instructions. Liver glycogen levels were determined by anthrone-sulfuric method. Contents of DA and 5-HT in the PVN were measured by ELISA kits.
1.3.4 Histological examination
Mice in experimental set 3 were used for histological examination. One hour after the last swimming training on the 10th day, the mice were anesthetized with sodium pentobarbital (50 mg/kg) injected intraperitoneally. Cold saline (50 mL) was infused quickly by ascending aorta to rinse the blood vessels, and then 50 mL of 4% (m/m)paraformaldehyde was infused for tissue pre-fixation. The brain and the right lobe of the liver was removed quickly and dropped into 30 mL 4% (m/m) paraformaldehyde to fi x for 3 h. Then the tissues were put into 30% (m/m) sucrose(4 ℃). After the tissues sank to the bottom, the frozen sections were prepared with 15 µm thickness and the paraventricular nucleus of PVN was located by the Mouse Brain in Stereotaxic Coordinates[8]. For c-fos staining, the sections of PVN were incubated in 0.05 mol/L phosphate buffered saline (PBS) containing 0.5% (m/m) Triton X-100 for 40 min at room temperature, blocked with normal goat serum for 1 h, and then incubated with rabbit anti-c-fos antibody (1:500,m/m) at 4 ℃ overnight. After rinsing in PBS, brain sections were incubated with goat biotin-labeled anti rabbit immunoglobulin (1:200,m/m), followed by visualization with DAB. When a brown reaction product appeared, the DAB staining reaction was terminated with water and counterstained with a hematoxylin staining solution. Finally photographed under BX50 microscope and DP50 digital camera.
Data analysis was carried out by SPSS 17 software. The results of each group were expressed in ±sand analyzed using one-way analysis of variance followed by Dunnett’s test for multiple comparisons. Differences atP < 0.05 were considered as signi fi cance.

Fig. 1 Effect of GSH on exhaustive swimming time of mice
The effect of GSH on swimming endurance of mice is shown in Fig. 1. The exhaustive swimming time in the EF group was significantly shorter than that in the NC group(P< 0.05), indicating that swimming training resulted in exhausting. Furthermore, the exhaustive swimming time in the MG and AG groups prolonged extremely significantly compared with NC and EF group (P< 0.01), respectively. In the three GSH groups, the exhaustive swimming time prolonged with the increase of dosage, and the exhaustive swimming time of the HG group was slightly more than that of the AG group, but there was no significant difference (P> 0.05).
Table 1 Effect of GSH on serum biochemical indexes in mice subjected to exhaustive swimming
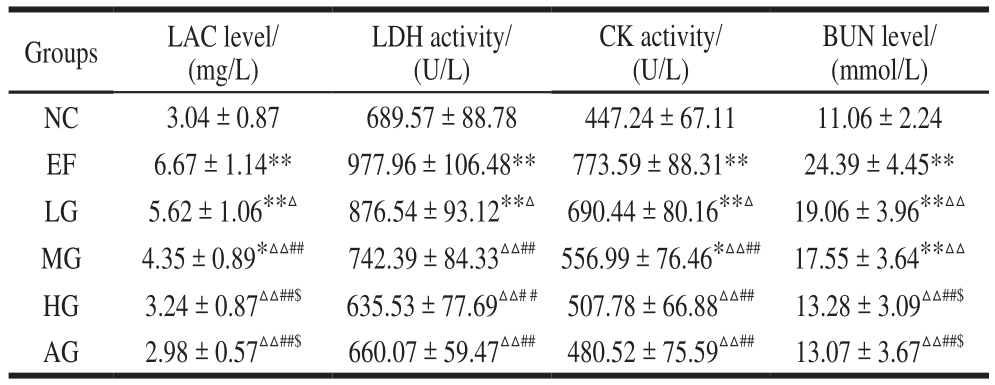
Note: Compared with the NC group, *. There is a significant difference among means (P < 0.05); **. There is an extremely significant difference among means (P < 0.01); Compared with EF group, △. There is a significant difference among means (P < 0.05); △△. There is an extremely significant difference among means (P < 0.01); Compared with LG group, #. There is a significant difference among means(P < 0.05); ##. There is an extremely significant difference among means (P < 0.01); Compared with MG group; $. There is a significant difference among means (P < 0.05); $$. There is an extremely significant difference among means (P < 0.01). The same below.
BUN level/(mmol/L)NC 3.04 ± 0.87 689.57 ± 88.78 447.24 ± 67.11 11.06 ± 2.24 EF 6.67 ± 1.14** 977.96 ± 106.48** 773.59 ± 88.31** 24.39 ± 4.45**LG 5.62 ± 1.06**△ 876.54 ± 93.12**△ 690.44 ± 80.16**△ 19.06 ± 3.96**△△MG 4.35 ± 0.89*△△## 742.39 ± 84.33△△## 556.99 ± 76.46*△△## 17.55 ± 3.64**△△HG 3.24 ± 0.87△△##$ 635.53 ± 77.69△△# # 507.78 ± 66.88△△## 13.28 ± 3.09△△##$AG 2.98 ± 0.57△△##$ 660.07 ± 59.47△△## 480.52 ± 75.59△△## 13.07 ± 3.67△△##$Groups LAC level/(mg/L)LDH activity/(U/L)CK activity/(U/L)
As shown in Table 1, the serum LDH, CK activity, and LAC, BUN levels increased extremely significantly in the EF group compared with that in the NC group (P< 0.01),which is related to the physical fatigue. Compared with the EF group, the levels in the three GSH groups decreased at different degrees, showing a dose-dependent manner(P< 0.05, P< 0.01). In addition, the serum LDH, CK activity, and BUN, LAC levels both in the HG and AG group had no significant difference with the NC group.
Table 2 Effect of GSH on liver biochemical indexes in mice subjected to exhaustive swimming
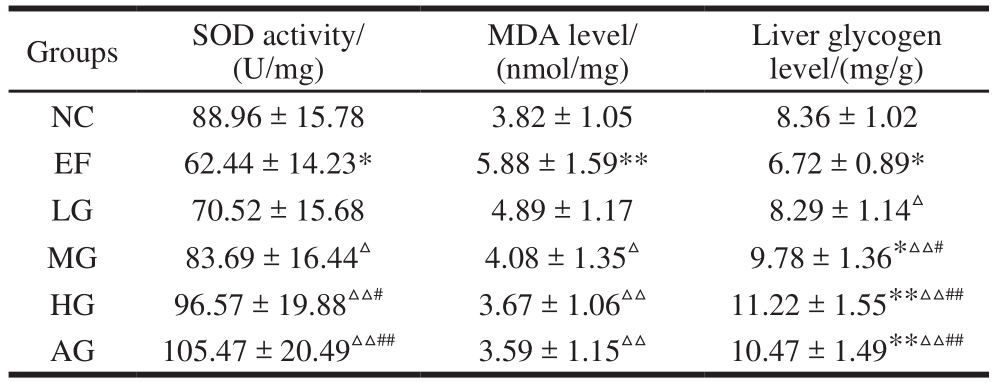
Groups SOD activity/(U/mg)Liver glycogen level/(mg/g)NC 88.96 ± 15.78 3.82 ± 1.05 8.36 ± 1.02 EF 62.44 ± 14.23* 5.88 ± 1.59** 6.72 ± 0.89*LG 70.52 ± 15.68 4.89 ± 1.17 8.29 ± 1.14△MG 83.69 ± 16.44△ 4.08 ± 1.35△ 9.78 ± 1.36*△△#HG 96.57 ± 19.88△△# 3.67 ± 1.06△△ 11.22 ± 1.55**△△##AG 105.47 ± 20.49△△## 3.59 ± 1.15△△ 10.47 ± 1.49**△△##MDA level/(nmol/mg)
As shown in Table 2, the activity of SOD in the liver tissue and the liver glycogen level decreased, while the level of MDA in the liver tissue increased significantly in the EF group (versus NC group,P< 0.05,P< 0.01). These results indicated that peroxidation injury and depletion of glycogen existed in the exercise fatigue induced by forced swimming. Compared with the EF group, the SOD activity and the content of liver glycogen increased, while MDA level decreased to different degrees in the three GSH groups in a dose-dependent manner. Among them,the changes in the MG group and the HG group are more significant (P< 0.05,P < 0.01 , Table 2). Amazingly, high dose of GSH increased liver glycogen level significantly(versus NC group,P< 0.01), which was beneficial for fatigue improvement. And there was no significant difference between HG group and AG group (P> 0.05).
Table 3 Effect of GSH on neurotransmitters in PVN of mice subjected to exhaustive swimming
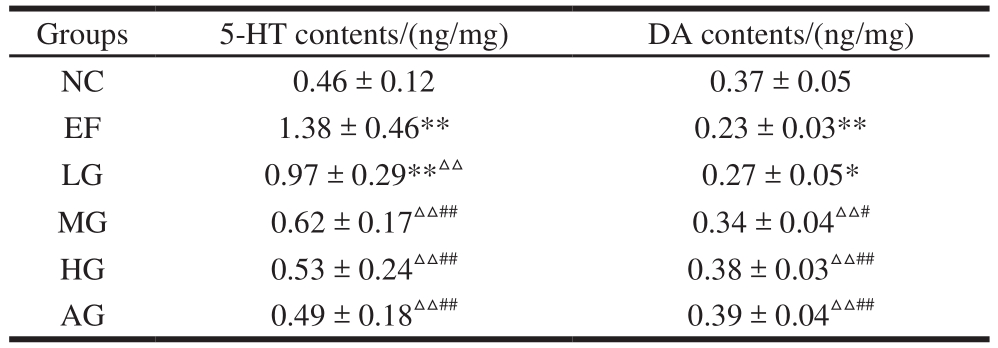
Groups 5-HT contents/(ng/mg) DA contents/(ng/mg)NC 0.46 ± 0.12 0.37 ± 0.05 EF 1.38 ± 0.46** 0.23 ± 0.03**LG 0.97 ± 0.29**△△ 0.27 ± 0.05*MG 0.62 ± 0.17△△## 0.34 ± 0.04△△#HG 0.53 ± 0.24△△## 0.38 ± 0.03△△##AG 0.49 ± 0.18△△## 0.39 ± 0.04△△##
As shown in Table 3, the contents of 5-HT in the PVN increased extremely significantly and the contents of DA in the PVN decreased extremely significantly in the EF group compared with the NC group (P < 0.01), indicating that the swimming exercise induced central fatigue in the mice.Compared with the EF group, the 5-HT content decreased,and the DA content increased in the three GSH groups in a dose-dependent manner (P < 0.05,P < 0.01). In addition,there was no significant difference between HG group and AG group (P> 0.05). Suggesting that GSH could relieve the central fatigue.
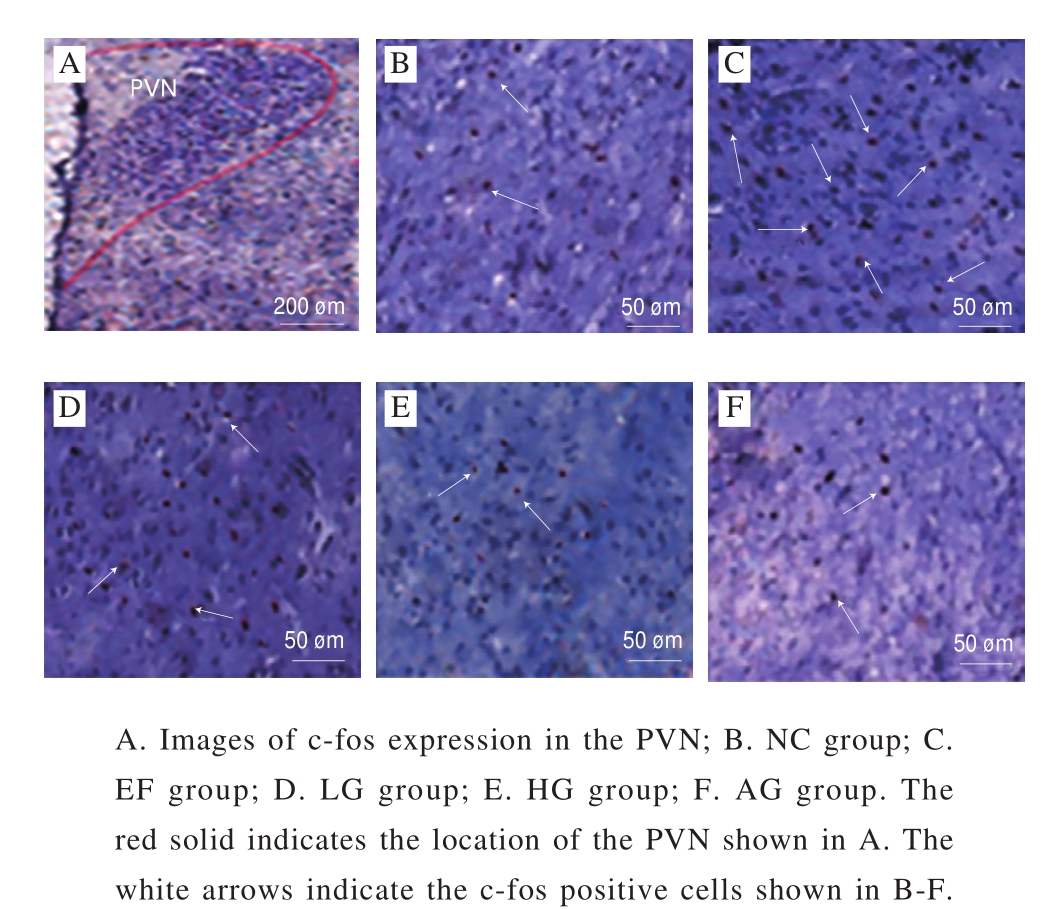
Fig. 2 Effect of GSH on c-fos expression in PVN of mice
As shown in the Fig. 2, c-fos positive cells were observed in the PVN in the EF group with a mark increase compared with the NC group. Otherwise, c-fos-IR cells decreased in the three GSH groups, indicating that GSH inhibit the neuron activity induced by exhaustive swimming.
This study aimed to evaluate the anti-fatigue effects of GSH and its possible mechanisms. Fatigue is a mental state of physical activity, which is characterized by a significant decrease in working ability and a low sense of achievement,usually accompanied by fatigue, sleepiness or irritability[9].Swimming test is the most common fatigue assessment method which can objectively reflect the physical fitness of the body. Our results showed that GSH prolonged the exercise endurance time in rats with a dose-dependent relationship when comparing with the normal control group.GSH showed obvious anti-fatigue effect in rats.
In addition to exercise endurance testing, quantification of blood and tissue-related indicators is another way to further explore the anti-fatigue property of GSH. The change of biochemical indexes such as liver glycogen content, LDH,SOD, CK activity, and LAC, BUN, MDA, DA and 5-HT levels are the most commonly used indicators in fatigue test.
Metabolic disorders conclude the consumption of energy sources (such as liver glycogen) and the production of metabolites (such as the accumulation of serum urea nitrogen,LAC and free radicals), all of which lead to fatigue[10].Glycogen is an extremely important substance that maintains the normal level of glucose in the blood. Adequate glycogen reserves are beneficial to improve endurance of body and reduce the decomposition of protein and nitrogenous compounds[11]. Therefore, the increasement of liver glycogen storage is essential to eliminate fatigue and improve exercise ability. In this study, the liver glycogen content in the GSH-treated rats were much higher than the control rats,indicating that GSH can increase glycogen storage to maintain the blood glucose level and provide energy materials to relieve physical fatigue.
As exercise time increases, lactate will be accumulated and pH value will decrease, leading to metabolic and physiological adverse effect on glycolytic processes and calcium ion release that associated with muscle contractions[12]. Therefore, after a long period of strenuous exercise, excessive accumulation of LAC in the body is also an important cause of exercise-induced fatigue. Through this study, we observed that GSH can inhibit the increase of LAC level, indicating that GSH can inhibit the accumulation of LAC or accelerate the clearance of LAC to reduce fatigue.
We assessed whether GSH can protect cells from injury by quantifying LDH and CK in the blood. As an important enzyme involved in glycolysis and gluconeogenesis, LDH has greatly promoted the oxidation of L-LAC and pyruvic acid reduction reaction[13]. Therefore, LDH often reflects the muscle activity accurately. Elevation of LDH in the serum indicates that the muscle injury is happening or has occurred.Similarly, serum CK is also an important clinical indicator for muscle injury and over exertion[14]. In the present study,serum levels of LDH and CK activity were significantly reduced in mice after exhaustive swimming treated by GSH.Above all, it can be speculated that the effect of GSH on fatigue may be due to its ability to protect cells from damage during intense exercise.
After long-term strenuous exercise, the body might obtain energy from proteins and amino acids instead of carbohydrates and fat. Under this circumstances, the protein and amino acid catabolites increase, which may reduce muscle strength and eventually lead to fatigue[15]. BUN is the fi nal product of protein metabolism, reflecting the breakdown of proteins and the degree of muscle contractility[16]. We found that the serum BUN levels in exhaustive swimming mice treated by GSH were significantly decreased. In conclusion, GSH can effectively reduce the metabolic rate of protein and increase the rate of metabolic waste clearance,improve the body’s ability to adapt to severe exercise and increase endurance.
Some studies suggest that oxidative stress is closely related to fatigue caused by exhaustive exercise[17]. Therefore,the effective removal of free radicals to inhibit of the oxidative stress is one of the most important ways to relieve exercise-induced fatigue. SOD is a naturally free radical scavenger in the body and an important antioxidant enzyme against reactive oxygen species. MDA is the end product of the oxidative stress response and is a sensitive indicator of lipid peroxidation in the body. The formation of oxygen free radicals is closely related to the content of MDA during exhaustive exercise, and the content of MDA reflects the degree of oxidative stress in the body. In our present study,GSH significantly increased SOD activity and decreased MDA content in exhaustive swimming mice, suggesting that GSH may reduce exercise-induced fatigue by increasing antioxidant activity and inhibiting lipid peroxidation.
In recent years, with the deepening awareness of fatigue, the mechanism of central fatigue has become a hot research topic of sports fatigue. Neumerous studies show that exercise-induced fatigue imposes effects on the neurotransmitter homeostasis, which might be the most crucial mechanism for exercise-induced fatigue[18]. Long term exercise induced central fatigue results in the accumulation or consumption of some neurotransmitters, such as monoamine neurotransmitters 5-HT and DA.
5-HT is an inhibitory neurotransmitter, which plays a variety of physiological roles in the central nervous system and the surrounding tissues. Studies have shown that the increase of 5-HT in the main brain areas such as PVN is related to the regulation of body temperature and the formation of fatigue[19].
DA is an important neurotransmitter in the pyramidal system that regulates body movement. DA is associated with a variety of physiological functions, such as arousal,reward and incentive, heat regulation and fatigue[20]. Our results showed that GSH treatment significantly decreased the content of 5-HT and increased the content of DA in the PVN of the fatigue mice after swimming. In conclusion, GSH exerted its anti-fatigue effect via improving the disturbance of neurotransmitters in PVN.
PVN is a heterogeneous nucleus involving in the regulation of many neurological functions. Recent studies have found that PVN is also involved in the regulation of body movement fatigue[21]. Modern medical studies have shown that chronic fatigue syndrome patients have hypothalamic-pituitary-adrenal axis (HPA) dysfunction[22],while PVN is an important part of HPA. In addition,the activation of hypothalamic neurons occurs mainly in the PVN, associating with increased blood glucose induced by exercise, lactate accumulation and epinephrine production[23].Furthermore, the PVN is an integration of the sympathetic nerve activities, which is considered to be involved in temperature control.
The c-fos expression is often regarded as a marker of neuron activation in response to neurotransmitters, hormones and other stimuli[24]. Normally, the c-fos expression is low in most cells, while increased substantially by stimulations such as stress, sexual behavior and pedal stimulation. In recent years, people have begun to study the relationship between c-fos and fatigue in order to reveal the mechanisms of fatigue[25]. The c-fos expression in PVN is also directly related to fatigue. Therefore, the effect of GSH on the c-fos expression in PVN of fatigue rats was observed in our experiment. The results showed that the c-fos-IR cells in the PVN were significantly increased in exhaustive swimming rats. The possible mechanism for the c-fos over-expression in the PVN is that exercise that acting as a stress indicatoraffects multiple parts of central nervous system. The increase of Ca2+level in the brain causes the discharge of neurons and the combination of calmodulin (CaM) to form Ca/CaM complex,which induces immediate expression of c-fos. In addition,Ca2+ also enhances the DA synthesis of the brain, and exercise cause the increase of neurotransmitters such as DA and 5-HT in the brain presynaptic membranes. These neurotransmitters,which act as first messengers of intercellular transmission,act on the DA receptor and 5-HT receptor that associated with nucleotide cyclase in the PVN. These receptors have an up-regulation effect on the second intracellular messengers cyclic adenine nucleotides (cAMP) which activates the c-fos gene expression[26]. However, after GSH treatment,the abnormal increase of c-fos in the PVN in the exhaustive swimming rats was reduced, indicating that GSH could inhibit the activation of PVN neurons.
In the present study, we explored the effect of GSH on exercise-induced fatigue in rats. Results showed that GSH significantly prolonged exhaustive exercise time, increasing liver glycogen level, SOD activity and hypothalamic DA contents and decreasing serum LDH, CK activity and LAC,BUN, liver MDA and hypothalamic 5-HT contents. In addition, GSH significantly reduced c-fos over-expression in the PVN induced by exercise fatigue. In summary,GSH exhibits obvious anti-fatigue effect in rats and has the potential to be developed for new anti-fatigue drugs.Otherwise, the detailed anti-fatigue mechanisms of GSH has yet to be further studied.
[1] LU S C. Glutathione synthesis[J]. Biochimica et Biophysica Acta,2013, 1830(5): 3143-3151. DOI:10.1016/j.bbagen.2012.09.008.
[2] PASTORE A, FEDERICI G, BERTINI E, et al. Analysis of glutathione:implication in redox and detoxification[J]. Clinica Chimica Acta,2003, 333(1): 19-39. DOI:10.1016/S0009-8981(03)00200-6.
[3] LUSHCHAK V I. Glutathione homeostasis and functions: potential targets for medical interventions[J]. Journal of Amino Acids, 2012,2012(5): 736837. DOI:10.1155/2012/736837.
[4] ZHAO Y Q, ZENG L, YANG Z S, et al. Anti-fatigue effect by peptide fraction from protein hydrolysate of croceine croaker (Pseudosciaena crocea) swim bladder through inhibiting the oxidative reactions including DNA damage[J]. Marine Drugs, 2016, 14(12): 221.DOI:10.3390/md14120221.
[5] XU M, LIANG R, LI Y, et al. Anti-fatigue effects of dietary nucleotides in mice[J]. Food & Nutrition Research, 2017, 61(1):1334485. DOI:10.1080/16546628.2017.1334485.
[6] UTHAYATHAS S, KARUPPAGOUNDER S S, TAMER S I, et al.Evaluation of neuroprotective and anti-fatigue effects of sildenafil[J].Life Sciences, 2007, 81(12): 988-992. DOI:10.1016/j.lfs.2007.07.018.
[7] XU C, LÜ J, LO Y M, et al. Effects of oat β-glucan on endurance exercise and its anti-fatigue properties in trained rats[J]. Carbohydrate Polymers,2013, 92(2): 1159-1165. DOI:10.1016/j.carbpol.2012.10.023.
[8] PAXINOS G, FRANKLIN K B J. The mouse brain in stereotaxic coordinates[M]. San Francisco: Academic Press, 2013: 87-90.
[9] CHEN H, HE X, LIU Y, et al. Extraction, purification and antifatigue activity of γ-aminobutyric acid from mulberry (Morus alba L.)leaves[J]. Scientific Reports, 2016, 6: 18933. DOI:10.1038/srep18933.
[10] SURHIO M M, WANG Y, FANG S, et al. Anti-fatigue activity of a Lachnum polysaccharide and its carboxymethylated derivative in mice[J]. Bioorganic & Medicinal Chemistry Letters, 2017, 27(20):4777-4780. DOI:10.1016/j.bmcl.2017.07.034.
[11] TAN W, YU K Q, LIU Y Y, et al. Anti-fatigue activity of polysaccharides extract from radix rehmanniae preparata[J].International Journal of Biological Macromolecules, 2012, 50: 59-62.DOI:10.1016/j.ijbiomac.2011.09.019.
[12] HUANG W C, LIN C I, CHIU C C, et al. Chicken essence improves exercise performance and ameliorates physical fatigue[J]. Nutrients,2014, 6(7): 2681-2696. DOI:10.3390/nu6072681.
[13] JIN H M, WEI P. Anti-fatigue properties of tartary buckwheat extracts in mice[J]. International Journal of Molecular Sciences, 2011, 12(8):4770-4780. DOI:10.3390/ijms12084770.
[14] CHEN Y M, LIN C L, LI W, et al. Sake protein supplementation affects exercise performance and biochemical profiles in powerexercise-trained mice[J]. Nutrients, 2016, 8(2): 106. DOI:10.3390/nu8020106.
[15] ZHONG L, ZHAO L, YANG F, et al. Evaluation of anti-fatigue property of the extruded product of cereal grains mixed with Cordyceps militaris on mice[J]. Journal of the International Society of Sports Nutrition, 2017, 14(1): 15. DOI:10.1186/s12970-017-0171-1.
[16] HSIAO C Y, CHEN Y M, HSU Y J, et al. Supplementation with Hualian No. 4 wild bitter gourd (Momordica charantia Linn. var.abbreviata ser.) extract increases anti-fatigue activities and enhances exercise performance in mice[J]. Journal of Veterinary Medical Science, 2017, 79(6): 110-119. DOI:10.1292/jvms.17-0079.
[17] KAN N W, HUANG W C, LIN W T, et al. Hepatoprotective effects of Ixora parviflora extract against exhaustive exercise-induced oxidative stress in mice[J]. Molecules, 2013, 18(9): 10721-10732. DOI:10.3390/molecules180910721.
[18] MEEUSEN R, WATSON P, HASEGAWA H, et al. Central fatigue:the serotonin hypothesis and beyond[J]. Sports Medicine, 2006,36(10): 881-909. DOI:10.2165/00007256-200636100-00006.
[19] RODRIGUES A G, SOARES D D, MARUBAYASHI U, et al.Heat loss during exercise is related to serotonin activity in the preoptic area[J]. Neuroreport, 2009, 20(8): 804-808. DOI:10.1097/WNR.0b013e32832b8c90.
[20] FOLEY T E, FLESHNER M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue[J]. Neuromolecular Medicine, 2008, 10(2): 67-80. DOI:10.1007/s12017-008-8032-3.
[21] HONG K, SHIN M S, LEE T H, et al. Treadmill exercise modulates nitric oxide synthase expression in the hypothalamus of streptozotocininduced diabetic rats[J]. Nutrition Research, 2004, 24(1): 95-105.DOI:10.1016/j.nutres.2003.09.009.
[22] LUAN X, SUN X R, GUO F F, et al. Lateral hypothalamic orexin-A-ergic projections to the arcuate nucleus modulate gastric function in vivo[J]. Journal of Neurochemistry, 2017, 143(6): 697-707.DOI:10.1111/jnc.14233.
[23] LIMA P M, SANTIAGO H P, SZAWKA R E, et al. Central blockade of nitric oxide transmission impairs exercise-induced neuronal activation in the PVN and reduces physical performance[J].Brain Research Bulletin, 2014, 108: 80-87. DOI:10.1016/j.brainresbull.2014.09.002.
[24] AKAZAWA K H, CUI Y, TANAKA M, et al. Mapping of regional brain activation in response to fatigue-load and recovery in rats with c-fos immunohistochemistry[J]. Neuroscience Research, 2010, 66(4):372-379. DOI:10.1016/j.neures.2009.12.009.
[25] FOLEY T E, BROOKS L R, GILLIGAN L J, et al. Brain activation patterns at exhaustion in rats that differ in inherent exercise capacity[J].PLoS ONE, 2012, 7(9): e45415. DOI:10.1371/journal.pone.0045415.
[26] STANCIU M, RADULOVIC J, SPIESS J. Phosphorylated cAMP response element binding protein in the mouse brain after fear conditioning: relationship to fos production[J]. Molecular Brain Research, 2001, 94(1/2): 15-24. DOI:10.1016/s0169-328x(01)00174-7.
谷胱甘肽对小鼠的抗疲劳作用及其机制