
Fig. 1 Chemical structures of rutin (A), quercetin (B), and kaempferol (C)
Mammals, such as humans, tend to overfeed themselves with food that are sweet and fatty especially. In 1986, the world average sugar consumption per capita was 56 g/d;However, it increased to 65 g/d in 2007[1]. The addictive fat consumption in people’s diets increased by 55% in the United States[1]. High-fructose diets or excessive fat intake may cause various metabolic syndrome (MS)-related diseases,including obesity, insulin resistance (IR), non-alcoholic fatty liver disease (NAFLD), and diabetes mellitus[2-5]. A previous study by the National Cholesterol Education Program(NCEP) showed that the morbidity of MS is about 24% in the US adult population, including 44% of those aged over 50 years old[6]. The prevalence rate of MS in China is about 21.3% in accordance with definitions of the revised NCEP ATPIII criteria[7]. Several studies revealed that patients with MS are susceptible to cardiovascular diseases[8]. In the past 30 years, the prevalence of type 2 diabetes (T2D)had tripled, and research has shown that patients with MS have five times higher risk of developing T2D compared with normal patients[9]. In addition, patients with MS have high liver fat contents[10], and 95% of obese patients develop NAFLD[11]. Pro-inflammatory cytokines, such as leptin,tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6),are induced by chronic consumption of high-fructose diet[12].Often accompanied by various lipid metabolic disorders, IR promotes visceral fat decomposition, which produces large amounts of free fatty acid (FFA) and increases the levels of liver total cholesterol (TC), total triglyceride (TG), and very low-density lipoprotein (VLDL)[5]. Increased VLDL provides more raw materials for low-density lipoprotein (LDL),thereby stimulating the synthesis of LDL while suppressing that of high-density lipoprotein (HDL)[5]. In consideration of the harmful consequences of MS, finding effective compounds to reduce the incidence of MS is significant.
Flavonoids have several beneficial activities, such as antioxidant activities, which depend on the compounds’different structure[13]. Quercetin, kaempferol, and rutin are common representative flavonoids which have similar molecular structures (Fig. 1). Treatment with flavonoids,such as quercetin or rutin, attenuates most MS-related diseases, including obesity, cardiovascular diseases, and NAFLD[14]. However, few studies have compared the effects of fl avonoids in alleviating MS.

Fig. 1 Chemical structures of rutin (A), quercetin (B), and kaempferol (C)
Accordingly, the present study compared the different effects of quercetin, kaempferol, and rutin on MS induced by high-fructose and high-fat diet (HFFD) in rats. The levels of serum biochemical indicators, hepatic antioxidant capacity,pro-inflammatory cytokines, and hepatic histological changes were determined.
Quercetin (98%), kaempferol (98%), and rutin (98%)Jingzhu Biotechnology Co. Ltd.; TG, TC, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), aspartate aminotransferase (AST),alanine aminotransferase (ALT), alkaline phosphatase (ALP),lactate dehydrogenase (LDH), creatine kinase (CK), total bilirubin (TBIL), and creatinine (Cre) commercial assay kits Biosino Bio-technology and Science Inc.; Total antioxidant capacity (TAOC), malondialdehyde (MDA),and FFA commercial assay kits Nanjing Jiancheng Bioengineering Institute; Plasma insulin radio-immunological assay kit HTA Co. Ltd.; Plasma adiponectin, leptin,TNF-α, C-reactive protein (CRP), and IL-6 commercial enzyme-linked immunosorbent assay (ELISA) kits Beijing Keyingmei Technology Co. Ltd.; Glucose Weifang Shengtai Pharmaceutical Co. Ltd.; Short-acting neutral insulin injection Jiangsu Wanbang Biochemical Pharmaceutical Co. Ltd.; Ethanol, chloroform and other chemicals were all of analytical grade.
The 50 male Sprague-Dawley (SD) rats (350-365 g)were supplied by Vital River Laboratory Animal Technology Co. Ltd. (Certificate SCXK (Jing) 2012-0001) and kept in a controlled environment ((23 ± 2) ℃, relative humidity(55 ± 5)%, normal diurnal variation, light: 8:00 a.m.-8:00 p.m.). Fifty SD rats were divided equally into 5 groups(n = 10) based on body mass and were housed individually after adaptation. The rats were fed by basal diet (NG), HFFD(MG), HFFD with quercetin (2.6 mmol/kg mb) (Q), HFFD with kaempferol (2.6 mmol/kg mb) (K) and HFFD with rutin(2.6 mmol/kg mb) (R) respectively for 13 weeks. There are many studies[14-18] indicated that 0.08% quercetin in diet(about 2.6 mmol/kg mb) was found to have a significant effect on MS related diseases. Meanwhile, some researches showed that 0.05% in diet (about 1.75 mmol/kg mb) is the dose of kaempferol that has a significant effect on MS[19-20].Moreover, study showed the dose of 100 mg/kg mb (about 4.06 mmol/kg mb) was found to be the most effective dose in modulating the changes during high fat diet induced pancreatic injury[21]. According to a lot of researches[14-23],we chose a relatively low, conservative but effective dose of these flavonoids. At the same time, in order to compare the intervening effects of quercetin, kaempferol, and rutin on MS, we chose an experimental concentration close to 2.6 mmol/kg mb with the same molar concentration. All of the experimental protocols and procedures were approved by the Ethics Committee of Beijing Key Laboratory of Functional Food from Plant Resources and conducted in accordance with the Animal Management Rules of the Ministry of Health of the People’s Republic of China. The ingredients of basal diet and HFFD were shown in Table 1.
Table 1 Composition of experimental diets

Nutritive index Basal diet HFFD Protein content/(g/kg) 200.0 188.8 Fat content/(g/kg) 45.0 264.3 Carbohydrate content/(g/kg) 500.0 389.9 Crude fi ber content/(g/kg) 38 38 Vitamins content/(g/kg) 2.10 2.09 Mineral content/(g/kg) 35.0 35.0 Water content/(g/kg) 81 80 Ash content/(g/kg) 65 65 Choline content/(g/kg) 0 2 Total energy/(kJ/g) 13.43 19.67
300 Automatic Analyzer Abbott Laboratories (USA);BX51 Miroscope Olympus Optical Co. Ltd. (Japan);One Touch Ultra Test Glucometer LifeScan Inc. (USA);SpectraMax M2e Microplate Reader Molecular Devices Co. Ltd. (USA).
1.3.1 Measurement of body and organ mass
During the experiment, body mass was recorded once a week. The mass of diet fed and diet left was recorded every day to calculate the food intake. Energy intake was calculated according to diet total energy and food intake.Food consumption was measured every day. After the 13-week experiment, animals were fasted overnight and sacrificed under ethyl ether inhalation anesthesia. After the measurement of abdominal circumference, removed liver,kidney, epididymal fat pads and perirenal fat pads, then weighed immediately and calculated the organ indices and body fat ratios.
1.3.2 Measurement of biochemistry index in blood
After overnight fasting (12-14 h), blood samples were taken from orbital venous by capillary tube under ethyl ether inhalation anesthesia, moreover, repeated the experiment every 3 weeks. Blood samples were kept at ambient temperature for 2 h and centrifuged at 4 000 × g for 10 min to obtain serum. Serum was transferred to Eppendorf tubes and stored at −80 ℃ for subsequent use. The concentrations of plasma TG, TC, LDL-C, HDL-C, FFA, ALT, AST, ALP,LDH, Cre, CK and TBIL levels were measured according to the instruction book of those assay kits by an Alcyon 300 automatic analyzer. The concentrations of serum TAOC, MDA levels were measured on SpectraMax M2e microplate reader. Serum insulin levels were analyzed by a radio-immunological assay kit at 13 weeks. The serum concentrations of leptin, adiponectin, TNF-α, CRP, and IL-6 levels were detected by commercial ELISA kits.
1.3.3 Measurement of oral glucose, insulin tolerance tests, glucose levels, and homeostasis model assessment of insulin resistance
Oral glucose (OGTT) and insulin tolerance tests(ITT) were measured by a One Touch Ultra test strips and glucometer. Fasting blood glucose levels were measured via tail blood of fasting rats (12 h) in oral glucose tolerance tests and the tail vein blood samples were taken before (0 min)and at subsequent time intervals of 30, 60, 90, and 120 min following glucose (2 g/kg mb) administration. For ITT, after 4 h of food deprivation following intraperitoneal injection of insulin (0.6 U/kg mb), the levels of fasting blood glucose were meassured and the tail vein blood samples were taken before(0 min) and at subsequent time intervals of 30, 50, 70 and 90 min. Calculated total areas under the curve (AUC) during OGTT and ITT, and homeostasis model assessment of insulin resistance (HOMA-IR) index (week 13) was carried out as previously described[17].
1.3.4 Histological observations
For the hematoxylin-eosin (HE) staining, fi xed hepatic tissue in 10% neutral buffered formalin, dehydrated and embedded in paraffin wax. Further, sliced and stained hepatic tissue with HE for determination of cellular necrosis, fat vacuole, and inflammatory cell infiltration. Meanwhile, frozen hepatic tissues in liquid nitrogen, sliced and stained with oil red O solution (0.5 g/100 mL, dissolved in isopropanol). All observations were carried out with a microscope.
1.3.5 Liver lipid measurement
Cut the hepatic tissue into pieces with ophthalmic operating scissors rapidly, then put them into a glass homogenizer for homogenizing and pestled with buffer (the ratio between homogenization buffer and hepatic tissue is 9:1,V/V). The liver tissue homogenate was removed to 0.5 mL centrifugal tubes and stored at −80 ℃ for further analysis. The whole experiment proceeded in ice-water bath method. Lipids from liver tissue sample were homogenized with chloroform-methanol mixture, 2:1 (V/V), and TC and TG of liver lipids were measured using corresponding commercial assay kits.
All data are presented as the ± s. Data graphics were conducted using Origin Pro 8.0 software. Differences were evaluated with a one-way analysis of variance followed by Tukey’s test performed by SPSS 13.0 statistical software.Signi fi cance was de fi ned as P < 0.05.
As shown in Fig. 2A, no significant difference in initial body mass was found among the fi ve groups. Body mass was significantly increased by HFFD in the MG relative to the NG(P < 0.05, Fig. 2B). Results showed that the fi nal body mass and body mass gain were attenuated with the consumption of kaempferol (P < 0.05, Fig. 2B and C). In addition, no significant difference in energy intake was observed among the five groups (P > 0.05, Fig. 2E). The liver index was significantly higher in the HFFD rats compared with the NG rats, whereas the addition of rutin significantly alleviated the liver index relative to the MG rats (P < 0.05, Fig. 2F).No significant difference in the kidney index was found among the five groups (P > 0.05, Fig. 2G). The perirenal and epididymal fat indices were significantly higher in the HFFD rats compared with the NG rats, but the addition of kaempferol significantly ameliorated fat accumulation in the perirenal and epididymal fat relative to the MG rats (P < 0.05,Fig. 2H and I).
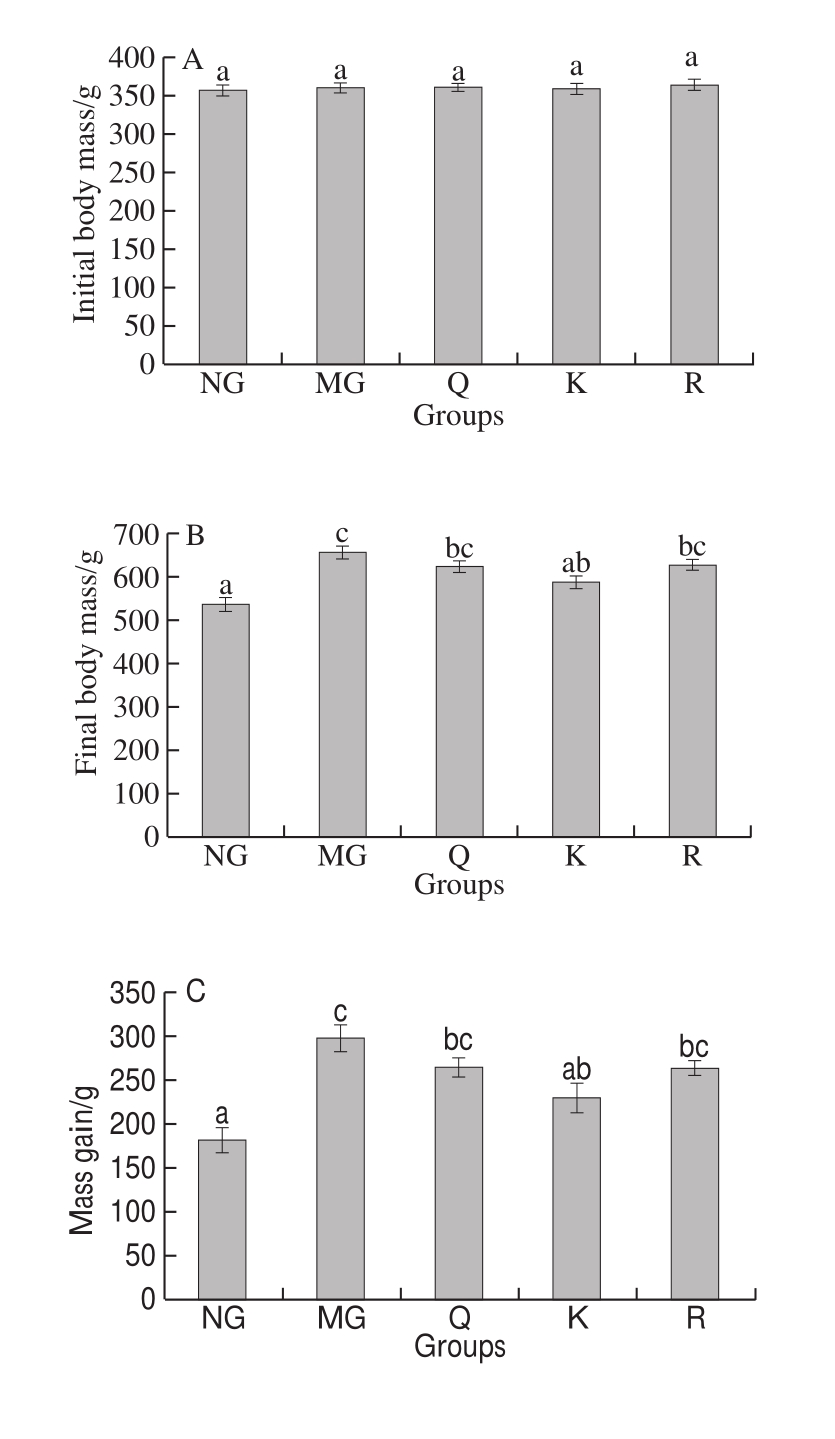

Fig. 2 Effects of quercetin, kaempferol and rutin on body mass, food intake, energy intake and organ indices
HFFD significantly increased hepatic TC level compared with NG; the addition of kaempferol or rutin alleviated hepatic TC level compared with MG, but no significant difference was found (P > 0.05, Fig. 3A). HFFD significantly increased hepatic TG level compared with NG, and the addition of kaempferol or rutin significantly decreased liver TG level compared with MG (P < 0.05, Fig. 3B). Kaempferol markedly reduced serum LDL-C level, whereas quercetin and rutin significantly increased serum HDL-C level compared with MG (P < 0.05, Fig. 3C and D). HFFD with kaempferol or rutin significantly decreased the levels of serum TC and FFA compared with MG (P < 0.05, Fig. 3E and G). In addition, the consumption of kaempferol significantly alleviated serum TG level compared with MG (P < 0.05, Fig. 3F).
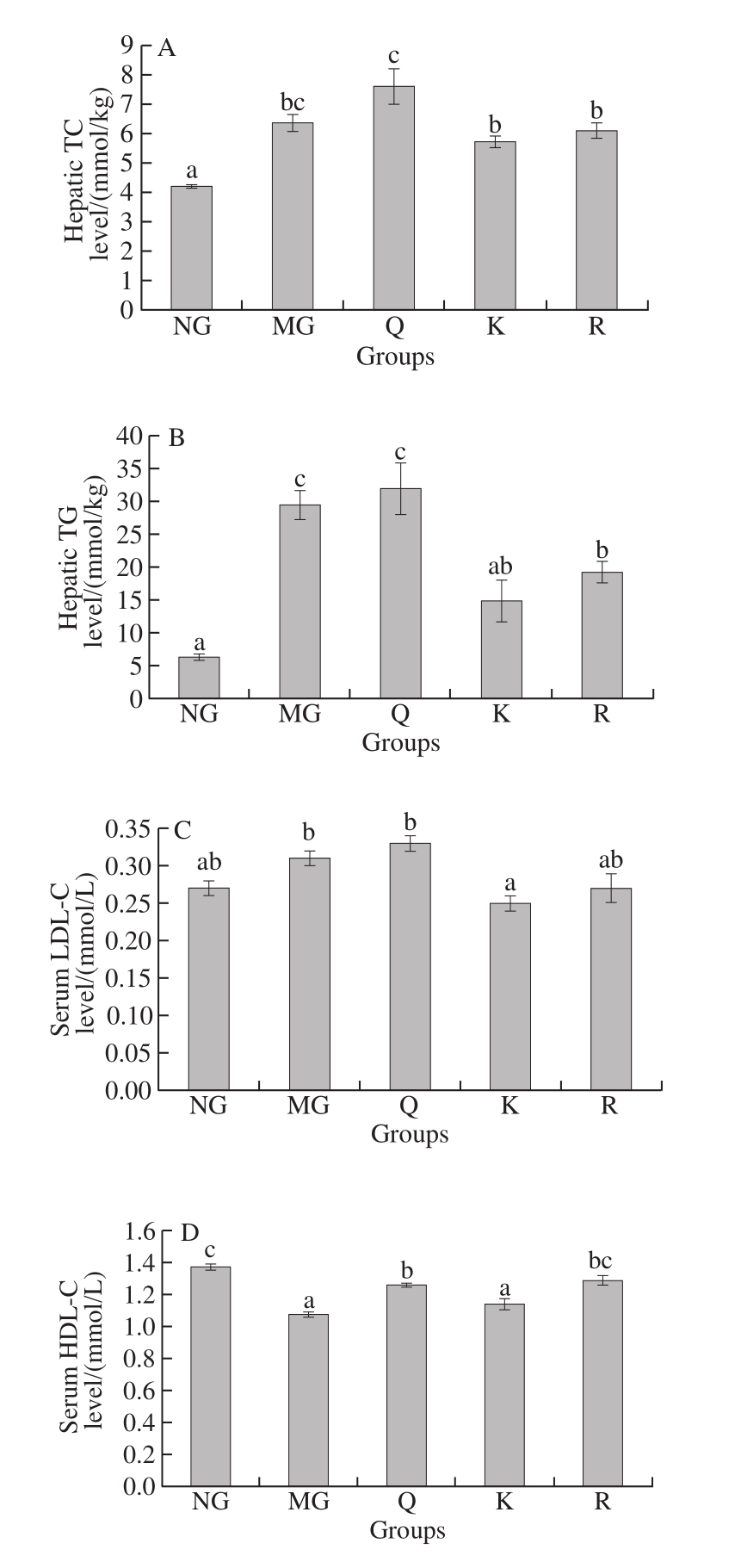

Fig. 3 Effects of quercetin, kaempferol and rutin on serum and hepatic lipid profiles


Fig. 4 Effects of quercetin, kaempferol and rutin on hepatic function
After 13 weeks of HFFD consumption, the activities of serum AST, ALP, LDH, and TBIL significantly increased in the MG rats compared with the NG rats (P < 0.05, Fig. 4).The addition of rutin markedly decreased the activities of serum AST activity compared with the MG (P < 0.05, Fig. 4A).ALT activity in the quercetin-treated group significantly decreased relative to that in MG (P < 0.05, Fig. 4B).Meanwhile, ALP activity in the quercetin, kaempferol, and rutin groups significantly increased relative to that in MG(P < 0.05, Fig. 4C). LDH activity was suppressed with the consumption of quercetin or kaempferol compared with the MG (P < 0.05, Fig. 4D). In addition, kaempferol or rutin significantly reduced the level of TBIL compared with the MG (P < 0.05, Fig. 4E).
After 13 weeks, the liver of the NG rats was completely normal, but small to medium red lipid droplets were found in the hepatocytes of the MG rats, indicating severe liver steatosis. After flavonoid intervention, the liver in the kaempferol-treated group almost returned to normal, and rutin could also alleviate liver steatosis histologically to some extent (Fig. 5).


Fig. 5 Histological examination of liver tissues (× 200)
HFFD significantly increased nephric Cre and serum CK levels compared with NG. However, the addition of rutin remarkably decreased these levels compared with MG(P < 0.05, Fig. 6).

Fig. 6 Effects of quercetin, kaempferol and rutin on nephric Cre and serum CK levels
HFFD consumption significantly increased fasting glucose levels and the AUC during OGTT and ITT compared with NG (P < 0.05, Fig. 7). Rutin significantly alleviated OGTT compared with the MG (P < 0.05, Fig. 7A), whereas kaempferol significantly improved ITT compared with the MG (P < 0.05, Fig. 7B). In addition, quercetin or kaempferol significantly decreased fasting glucose levels compared with MG (P < 0.05, Fig. 7C). However, no significant difference in the level of insulin level was found (P > 0.05, Fig. 7D).Compared with MG, quercetin or rutin markedly reduced the HOMA-IR index (P < 0.05, Fig. 7E).

Fig. 7 Effects of quercetin, kaempferol and rutin on AUC during OGTT and ITT, fasting glucose, fasting insulin and HOMA-IR index

Fig. 8 Effects of quercetin, kaempferol and rutin on serum antioxidant capacity
TAOC was remarkably decreased and MDA level was significantly increased in the HFFD rats compared with the NG rats (P < 0.05, Fig. 8). The three fl avonoid groups showed a rising tendency in TAOC and a decreasing tendency in MDA levels compared with MG, but no significant difference was found (P > 0.05).
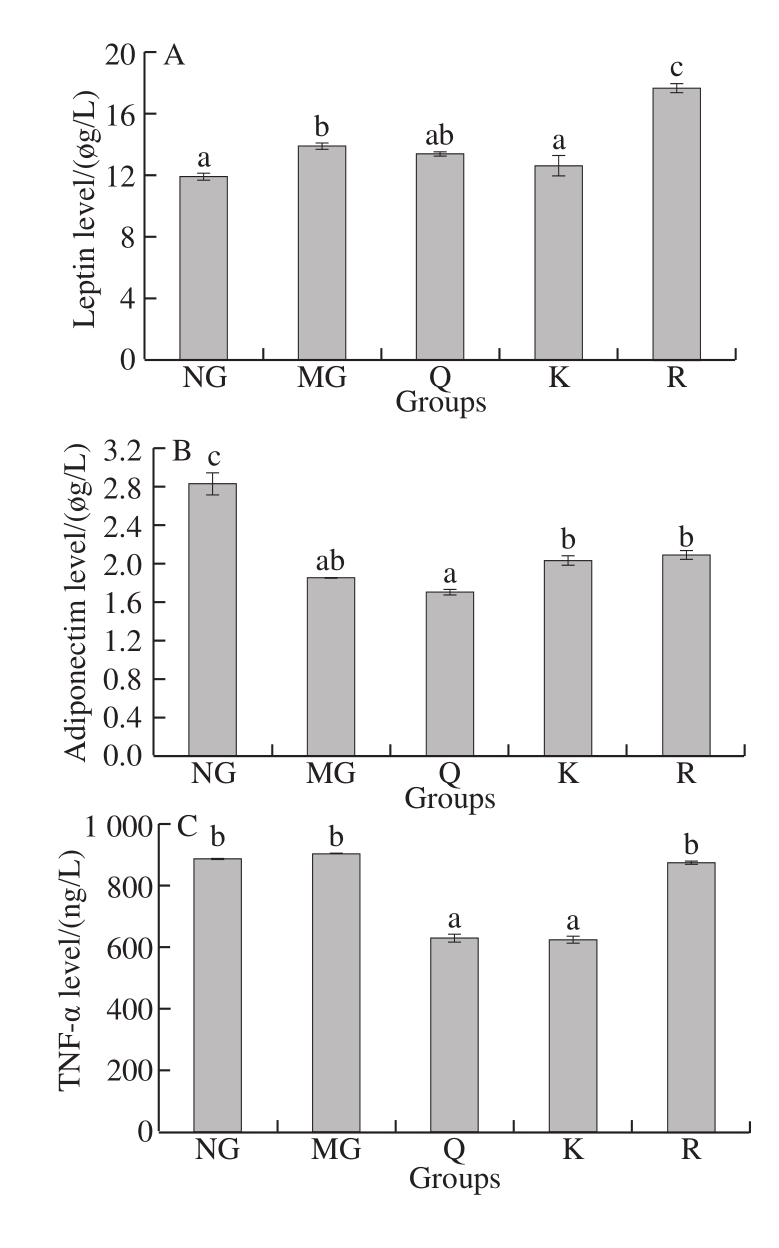
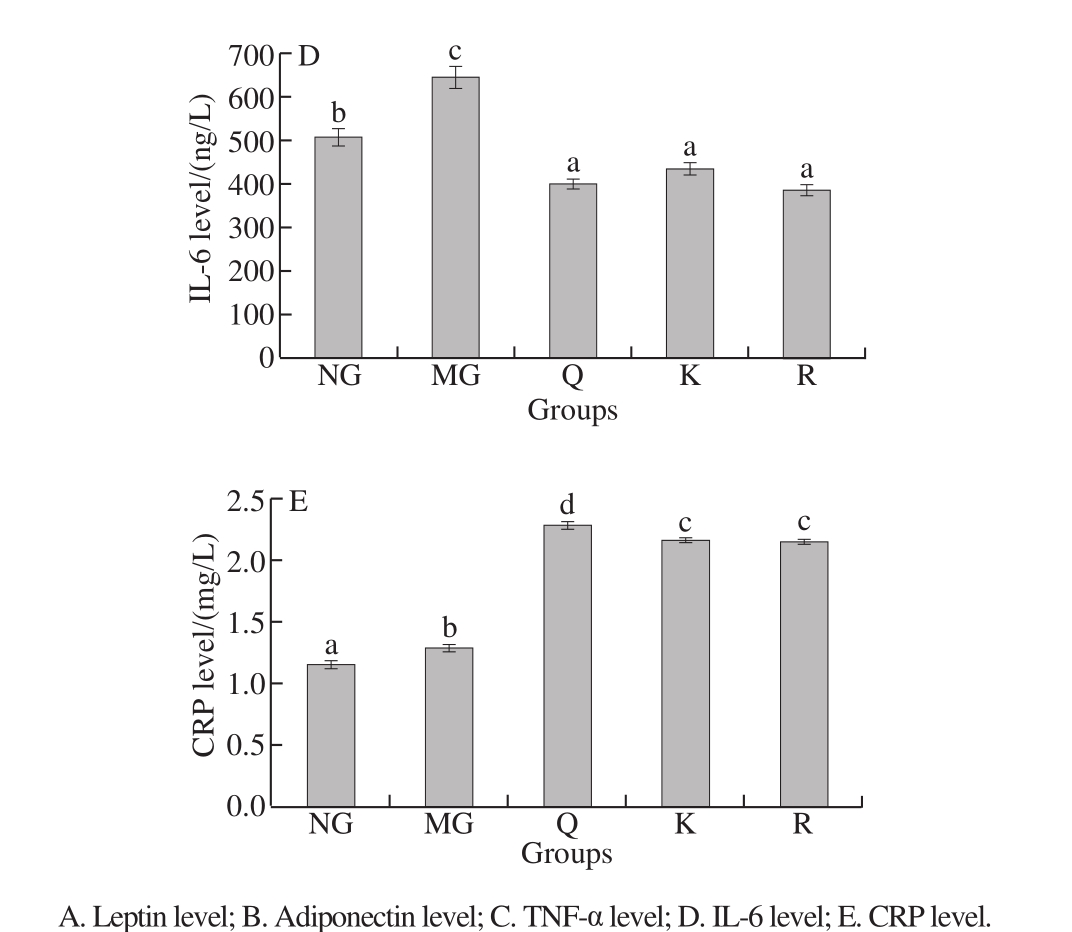
Fig. 9 Effects of quercetin, kaempferol and rutin on serum cytokine levels
Adiponectin level significantly decreased and leptin,IL-6, and CRP levels significantly increased in the HFFD rats compared with the NG rats (P < 0.05, Fig. 9). The level of leptin significantly decreased in the kaempferol-treated group compared with MG (P < 0.05, Fig. 9A). The level of adiponectin increased in the kaempferol- and rutin-treated group compared with MG, but no significant difference was found (P > 0.05, Fig. 9B). The level of TNF-α was significantly alleviated in the quercetin- or kaempferol-treated group compared with MG (P < 0.05, Fig. 9C). In addition,treatment with quercetin, kaempferol, or rutin significantly decreased the level of IL-6 compared with MG(P < 0.05, Fig. 9D).
MS is a growing and pres sing problem worldwide[2,6].Flavonoid-rich foods as well as pure flavonoids have great potential for preventing and/or ameliorating MS and MS-associated diseases[24]. In the present study, HFFD was used to induce MS in rats. Moreover, the function of quercetin, kaempferol,and rutin was principally demonstrated in this study. All three fl avonoids showed evident effects on obesity, lipid metabolism,carbohydrate metabolism, and oxidative stress.
Results showed that HFFD caused obesity of rats,the body mass gain, perirenal fat index and epididymal fat index were significantly increased by HFFD in the MG rats compared to the NG rats (Fig. 2). The dietary intake of quercetin, kaempferol, and rutin reduced body mass gain,as well as visceral and liver fat accumulation, and improved systemic parameters related to MS (Fig. 2). After 13-week feeding with HFFD, results showed that the body mass control capacity of kaempferol was more excellent than those of quercetin and rutin, and kaempferol might, to some extent,suppress the appetite (Fig. 2). Chang et al[25] demonstrated the similar conclusion that kaempferol reduced the accumulation of visceral fat and improved hyperlipidemia in HFFD-fed rats.Quercetin, kaempferol, and rutin reduced fat accumulation probably by decreasing oxidative stress and upregulating peroxisome proliferator-activated receptor (PPAR). One study indicated that quercetin may reduce fat accumulation by decreasing oxidative stress and increasing PPARα expression, as well as the improvement of gene expression related to steatosis in the liver[17]. Another study indicated that the molecular mechanism of polygonati rhizoma and kaempferol activity is related to the regulation of PPARγ expression and activation[18]. Some evidence indicated that the mRNA expression levels of adipogenic transcription factors such as PPARγ and CCAAT/enhancer-binding protein-α in 3T3-L1 cells are remarkably downregulated by rutin treatment[26].
Adipocytes, especially in obese individuals, secrete a number of pro-inflammatory cytokines, some of which inhibit insulin signaling directly[27]. Numerous cytokines,such as TNF-α and IL-6, cause IR in adipocytes[28]. Some studies suggested that leptin could serve as a valuable marker for predicting acute inflammation in individuals with obesity and diabetes due to the relationship of leptin with acute and chronic inflammation[29]. Previous reports have also suggested that low levels of adiponectin could be markers of MS,T2D, and IR[17,30]. In the present study, HFFD significantly decreased the level of adiponectin and remarkably increased the levels of leptin, IL-6, and CRP in MG rats compared with NG (Fig. 9). Previous studies indicated that plasma adiponectin levels are significantly increased and TNF-α levels are reduced after feeding with flavonoids such as quercetin[17]. Our results indicated that kaempferol, compared to quercetin and rutin, showed more positive effects in increasing the level of adiponectin and decreasing the levels of leptin, IL-6, and CRP (Fig. 9), it is a new discovery based on existing research[31].
When the production of reactive oxygen species (ROS)overwhelms the intrinsic antioxidant defense in the body, the pro-oxidant-antioxidant balance is disrupted and oxidative stress is subsequently generated[32]. ROS play crucial roles in inducing IR and promote the secretion of inflammatory factors[33]. ROS could react with polyunsaturated fatty acids to produce lipid peroxidation products like MDA[34],and elevated MDA levels could trigger various diseases associated with free radical damage[35]. Determination of these biomarkers has been widely applied to assess lipoperoxidation in biological and medical sciences[34].Fig. 8 indicated that HFFD increased MDA level and reduced TAOC, and the three flavonoid groups showed a rising tendency in TAOC and a decreasing tendency in MDA levels compared with MG, but no significant difference was found.Meanwhile, Shen et al[36] indicated that quercetin can raise TAOC, reduce the MDA generation.
The pathophysiology of MS was widely accepted by the hypothesis of IR[6]. IR can cause severe lipid metabolic disorders, resulting in the elevation of TG levels and decrease in LDL receptor levels[37]. However, it can also increase FFAs and lower lipoprotein lipase activity, further causing elevated hepatic FFAs and VLDL[11]. Insulin is also a direct moderator of glucose metabolism in the body and plays an important role in lipid metabolism[38]. Most individuals with these pre-diabetic states, such as impaired fasting glucose and impaired glucose tolerance, eventually develop diabetes[39].Another study proposed that a lowered beta-cell mass through genetic or beta-cell cytotoxic factors is a predisposing factor for glucose intolerance[40]. After 13 weeks of HFFD consumption, the activities of hepatic TC and TG significantly increased in the MG rats compared with the NG rats (Fig. 3). Meanwhile, HFFD significantly increased fasting glucose levels and the AUC during OGTT and ITT compared with NG (Fig. 7). Study demonstrated that quercetin significantly decreased serum glucose,FFA levels, and HOMA-IR index, compared with control group[25]. However, the present results showed that kaempferol and rutin regulated IR and reduced the levels of FFAs, serum TC, serum TG, hepatic TC, and hepatic TG more positive than quercetin (Fig. 3). Moreover, we found that quercetin and rutin can significantly reduce HOMA-IR index (Fig. 7). This finding suggests that flavonoids can reduce the level of fasting hyperglycemia by ameliorating hepatic IR.
Study presented that ALT, AST, ALP and LDH activities are serum marker enzymes which are the common biomarkers of liver damage[41]. After 13 weeks of HFFD consumption, the activities of serum AST, ALP, LDH,and TBIL significantly increased in the MG rats compared with the NG rats (P < 0.05, Fig. 4), indicating that the liver function of the MG rats has been seriously impaired. Results showed rutin reduced the activities of serum AST activity and the content of TBIL in serum, moreover, quercetin is more helpful in reduced the activities of serum ALT. Some studies indicated that quercetin affected the serum activities of ALT,AST, ALP, and LDH probably by protecting hepatocyte[42].Researches indicated that ALT is a cytosolic enzyme mainly present in the cell cytoplasm, and AST is a mitochondrial enzyme, which is released from the liver and other organs in the body[43].
In comparison, some reports used fructose alone to feed rats and to illustrate the effect of fl avonoids on glucose and lipid metabolism and oxidative stress[14,22,44-45]. However, we used HFFD to induce MS in rats and determined the effects of three fl avonoids with similar molecular structures on MS.
Quercetin reduced the level of fasting hyperglycemia and increased the level of HDL-C significantly. Among the three flavonoids, kaempferol was the most effective in alleviating body mass and fat accumulation, and rutin showed the best effect in decreasing the concentration of Cre and the serum level of CK. This study provided clear evidence that quercetin, kaempferol, and rutin can be used in the prevention of MS-related diseases, but these compounds play different biological roles in HFFD-induced MS despite having similar structures.
[1] TAPPY L, LÊ K A. Metabolic effects of fructose and the worldwide increase in obesity[J]. Physiological Reviews, 2010, 90(1): 23-46.DOI:10.1152/physrev.00019.2009.
[2] FAEH D, MINEHIRA K, SCHWARZ J M, et al. Effect of fructose overfeeding and fi sh oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men[J]. Diabetes, 2005, 54(7): 1907-1913. DOI:10.2337/diabetes.54.7.1907.
[3] TRAN L T, YUEN V G, MCNEILL J H. The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension[J]. Molecular and Cellular Biochemistry, 2009, 332(1/2):145-159. DOI:10.1007/s11010-009-0184-4.
[4] MURASE T, MIZUNO T, OMACHI T, et al. Dietary diacylglycerol suppresses high fat and high sucrose diet-induced body fat accumulation in C57BL/6J mice[J]. Journal of Lipid Research, 2001,42(3): 372-378.
[5] OAKES N D, COONEY G J, CAMILLERI S, et al. Mechanisms of liver and muscle insulin resistance induced by chronic highfat feeding[J]. Diabetes, 1997, 46(11): 1768-1774. DOI:10.2337/diab.46.11.1768.
[6] TONKIN A. The metabolic syndrome: a growing problem[J].European Heart Journal Supplements, 2004, 6(Suppl A): 37-42.DOI:10.1016/j.ehjsup.2004.01.009.
[7] XI B, HE D, HU Y, et al. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: the China Health and Nutrition Survey in 2009[J]. Preventive Medicine, 2013, 57(6): 867-871. DOI:10.1016/j.ypmed.2013.09.023.
[8] STERN M P, WILLIAMS K, HUNT K J. Impact of diabetes/metabolic syndrome in patients with established cardiovascular disease[J].Atherosclerosis Supplements, 2005, 6(2): 3-6. DOI:10.1016/j.atheroscl erosissup.2005.02.002.
[9] FORD E S, LI C, SATTAR N. Metabolic syndrome and incident diabetes: current state of the evidence[J]. Diabetes Care, 2008, 31(9):1898-1904. DOI:10.2337/dc08-0423.
[10] KOTRONEN A, YKI-JÄRVINEN H. Fatty liver: a novel component of the metabolic syndrome[J]. Arteriosclerosis Thrombosis and Vascular Biology, 2008, 28(1): 27-38. DOI:10.1161/ATVBAHA.107.147538.
[11] TANGVARASITTICHAI S. Oxidative stress, insulin resistance,dyslipidemia and type 2 diabetes mellitus[J]. World Journal of Diabetes, 2015, 6(3): 456-480. DOI:10.4239/wjd.v6.i3.456.
[12] CHENG Q, ZHANG X F, WANG O, et al. Anti-diabetic effects of the ethanol extract of a functional formula diet in mice fed with a fructose/fat-rich combination diet[J]. Journal of the Science of Food and Agriculture, 2015, 95(2): 401-408. DOI:10.1002/jsfa.6737.
[13] BROWN E J, KHODR H, HIDER C R, et al. Structural dependence of fl avonoid interactions with Cu2+ ions: implications for their antioxidant properties[J]. Biochemical Journal, 1998, 330(3): 1173-1178.DOI:10.1063/1.118214.
[14] PANCHAL S K, POUDYAL H, BROWN L. Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats[J]. Journal of Nutrition, 2012, 142(6):1026-1032. DOI:10.3945/ jn.111.157263.
[15] CHOI H N, JEONG S M, HUH G H, et al. Quercetin ameliorates insulin sensitivity and liver steatosis partly by increasing adiponectin expression in ob/ob mice[J]. Food Science & Biotechnology, 2015,24(1): 273-279. DOI:10.1007/s10068-015-0036-9.
[16] KIM J H, KANG M J, CHOI H N, et al. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus[J]. Nutrition Research & Practice, 2011, 5(2): 107-111.DOI:10.4162/nrp.2011.5.2.107.
[17] MASUKO K, MASUMOTO S, AKIMOTO Y, et al. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a Western-style diet in C57/BL6J mice[J]. Molecular Nutrition & Food Research, 2011, 55(4): 530-540. DOI:10.1002/mnfr.201000392.
[18] JEONG S M, KANG M J, CHOI H N, et al. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice[J]. Nutrition Research and Practice, 2012,6(3): 201-207. DOI:10.4162/nrp.2012.6.3.201.
[19] SUCHAL K, MALIK S, KHAN S I, et al. Molecular pathways involved in the amelioration of myocardial injury in diabetic rats by kaempferol[J]. International Journal of Molecular Sciences, 2017,18(5): 1001-1017. DOI:10.3390/ijms18051001.
[20] LIU G B, LIU Y G, SUN C, et al. Effects of kaempferol on glycolipid metabolism and insulin resistance in rats with type 2 diabetes[J].Journal of Clinical Medicine in Practice, 2012, 16(9): 1-3.
[21] ARUNA R, GEETHA A, SUGUNA P. Rutin modulates ethanol and high fat diet-induced inflammatory changes in pancreas: a dose response study in rats[J]. International Journal of Pharmacy &Pharmaceutical Sciences, 2012, 4(4): 409-414.
[22] PANCHAL S K, POUDYAL H, ARUMUGAM T V, et al. Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats[J]. Journal of Nutrition, 2011, 141(6): 1062-1069. DOI:10.3945/jn.111.137877.
[23] AL-REJAIE S S, ALEISA A M, SAYED-AHMED M M, et al.Protective effect of rutin on the antioxidant genes expression in hypercholestrolemic male Westar rat[J]. BMC Complementary and Alternative Medicine, 2013, 13(1): 136. DOI:10.1186/1472-6882-13-136.
[24] GALLEANO M, CALABRO V, PRINCE P D, et al. Flavonoids and metabolic syndrome[J]. Annals of the New York Academy of Sciences,2012, 1259(1): 87-94. DOI:10.1111/j.1749-6632.2012.06511.x.
[25] CHANG C J, TZENG T F, LIOU S S, et al. Kaempferol regulates the lipid-profile in high-fat diet-fed rats through an increase in hepatic PPARα levels[J]. Planta Medica, 2011, 77(17): 1876-1882.DOI:10.1055/s-0031-1279992.
[26] CHOI I, PARK Y, CHOI H, et al. Anti-adipogenic activity of rutin in 3T3-L1 cells and mice fed with high-fat diet[J]. BioFactors, 2010,26(4): 273-281. DOI:10.1002/biof.5520260405.
[27] DUNCAN B B, SCHMIDT M I, PANKOW J S, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study[J]. Diabetes, 2003, 52(7):1799-1805. DOI:10.2337/diabetes.52.7.1799.
[28] XU H Y, BARNES G T, YANG Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance[J]. Journal of Clinical Investigation, 2003, 112(12): 1821-1830. DOI:10.1172/JCI200319451.
[29] MIRZA S, HOSSAIN M, MATHEWS C, et al. Type 2-diabetes is associated with elevated levels of TNF-α, IL-6 and adiponectin and low levels of leptin in a population of Mexican American: a crosssectional study[J]. Cytokine, 2012, 57(1): 136-142. DOI:10.1016/j.cyto.2011.09.029.
[30] LU J Y, HUANG K C, CHANG L C, et al. Adiponectin: a biomarker of obesity-induced insulin resistance in adipose tissue and beyond[J].Journal of Biomedical Science, 2008, 15(5): 565-576. DOI:10.1007/s11373-008-9261-z.
[31] PALACZ-WROBEL M, BORKOWSKA P, PAUL-SAMOJEDNY M,et al. Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha(TNF-α) and interleukin-10 (IL-10) in RAW-264.7 macrophages[J].Biomedicine & Pharmacotherapy, 2017, 93: 1205-1212. DOI:10.1016/j.biopha.2017.07.054.
[32] MITTLER R. Oxidative stress, antioxidants and stress tolerance[J].Trends in Plant Science, 2002, 7(9): 405-410. DOI:10.1016/S1360-1385(02)02312-9.
[33] WANG O, LIU J, CHENG Q, et al. Effects of ferulic acid and gammaoryzanol on high-fat and high-fructose diet-induced metabolic syndrome in rats[J]. PLoS ONE, 2015, 10(2): e0118135. DOI:10.1371/journal.pone.0118135.
[34] MATEOS R, LECUMBERRI E, RAMOS S, et al. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress. application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits[J]. Journal of Chromatography B,2005, 827(1): 76-82. DOI:10.1016/j.jchromb.2005.06.035.
[35] SUTTNAR J, MÁSOVÁ L, DYR J E. Influence of citrate and EDTA anticoagulants on plasma malondialdehyde concentrations estimated by high-performance liquid chromatography[J]. Journal of Chromatography B Biomedical Sciences & Applications, 2001,751(1): 193-197. DOI:10.1016/S0378-4347(00)00453-9.
[36] SHEN Q H, QIN Z M, KONG R X. Study on protection function of quercetin on ischemia-reperfusion injury of liver and its mechanism[J].International Forum on Bioinformatics and Medical Engineering,2015, 2015: 68-72. DOI:10.2991/bme-15.2015.13.
[37] COPPACK S W, EVANS R D, FISHER R M, et al. Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal[J]. Metabolism-clinical & Experimental, 1992, 41(3): 264-272.DOI:10.1016/0026-0495(92)90269-G.
[38] SALTIEL A R, KAHN C R. Insulin signalling and the regulation of glucose and lipid metabolism[J]. Nature, 2001, 414: 799-806.DOI:10.1038/414799a.
[39] NATHAN D M, DAVIDSON M B, DEFRONZO R A, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care[J]. Diabetes Care, 2007, 30(3): 753-759. DOI:10.2337/dc07-9920.
[40] LEAHY J L. Pathogenesis of type 2 diabetes mellitus[J]. Archives of Medical Research, 2005, 36(3): 197-209. DOI:10.1016/j.arcmed.2005.01.003.
[41] SONG Z Y, ZHOU Z X, DEACIUC I, et al. Inhibition of adiponectin production by homocysteine: a potential mechanism for alcoholic liver disease[J]. Hepatology, 2010, 47(3): 867-879. DOI:10.1002/hep.22074.
[42] ZHANG Y, DENG Z S, ZHANG Y D. Protective effect of nanoscale quercetin liposome on hepatic injury in rats[J]. China Journal of Modern Medicine, 2012, 22(16): 33-36.
[43] HAN Y, XU Q, HU J N, et al. Maltol, a food flavoring agent,attenuates acute alcohol-induced oxidative damage in mice[J].Nutrients, 2015, 7(1): 682-696. DOI:10.3390/nu7010682.
[44] HOANG M H, JIA Y, MOK B, et al. Kaempferol ameliorates symptoms of metabolic syndrome by regulating activities of liver X receptor-β[J]. Journal of Nutritional Biochemistry, 2015, 26(8):868-875. DOI:10.1016/j.jnutbio.2015.03.005v.
[45] ABO-YOUSSEF A M. Protective effect of rosiglitazone, quercetin,and their combination on fructose-induced metabolic syndrome in rats[J]. Indian Journal of Pharmacology, 2015, 47(6): 620-626.DOI:10.4103/0253-7613.169577.
程倩(1986—)(ORCID: 0000-0003-1520-4121),女,工程师,博士,研究方向为功能性食品。E-mail: chqian001@163.com
周峰(1980—)(ORCID: 0000-0003-0665-8076),男,副教授,博士,研究方向为功能性食品。E-mail: zf@cau.edu.cn
槲皮素、山柰酚和芦丁对高果糖和高脂饮食诱导的大鼠代谢综合征的影响