纳米颗粒在侧流免疫层析技术中的应用研究进展
田亚晨,王淑娟,马 兰,谢曼曼,许东坡,刘 程,丁承超,郭 亮,方水琴,王 翔,邱景璇,董庆利,刘 箐*
(上海理工大学医疗器械与食品学院,上海 200093)
摘 要: 侧流免疫层析技术(lateral flow immunoassay,LFIA)有效地结合了色谱分析卓越的分离能力和免疫分析的高度特异性,加之其便携性,使其为需要灵敏、定量的现场检测提供了一个理想的平台。LFIA中,标记物是影响其灵敏度的关键因素之一。目前,LFIA的检测性能主要通过使用纳米颗粒标记物来改善。纳米颗粒标记物按材料可分为有色纳米颗粒、发光纳米颗粒以及磁性纳米颗粒等。本文旨在归纳总结纳米颗粒作为标记物在LFIA中的最新研究进展,详尽解析了各类纳米颗粒对试纸条分析性能的改善情况,以期为研究者在设计试纸条时对适宜纳米颗粒的选择提供有力的技术支持。
关键词: 纳米颗粒;标记物;侧流免疫层析技术
侧流免疫层析(lateral flow immunoassay,LFIA)技术是一种结合了色谱分析和免疫反应原理的固相免疫层析技术,最初称为“溶胶颗粒免疫测定”,是一种典型的现场检测技术。标记材料同时承担LFIA检测结果的“可视化”和“抗体标记”两大功能,对该方法的灵敏度、检测限(limit of detection,LOD)等性能至关重要。以20~30 nm金纳米颗粒作为标记物的LFIA,由于亮度不足导致灵敏度低[1-2]。而新型纳米颗粒用于生物分析标记物质,极大地改善了标记物性能,显著提升了现有分析方法的灵敏度及特异性。因此,新型纳米颗粒标记物可提高传统LFIA的分析性能,并广泛应用于实际检测中。本文就各种新型纳米颗粒标记物的优缺点及各类纳米颗粒对试纸条性能的改善进行详尽分析,以期对LFIA技术的发展提供借鉴。
1 纳米颗粒简介
纳米颗粒一般是指三维空间尺寸至少有一维尺寸在1~100 nm范围内的超微粒子。纳米颗粒一般具有表面效应、小尺寸效应、量子效应、宏观量子隧道效应等特性[3]。与正常粒子相比,纳米颗粒具有大的比表面积,其光、热、磁敏感特性和表面稳定性较高,拥有“21世纪最有前途材料”的美誉[4]。纳米材料由于具有特殊的结构层次、较强的吸附能力、良好的定向性能、生物相容性以及结构相容性(酶、抗原、抗体以及生物分子受体具有和纳米材料相似的尺寸约2~20 nm)等优势,在抗原抗体的标记上具有很大的应用潜能,是LFIA最常用的抗体标记材料。另外LFIA材料还要求具有功能广、响应时间短、灵敏度高、检测范围广等优点,纳米材料能较好地满足上述要求。
2 纳米颗粒分类及优缺点
目前,LFIA的分析灵敏度主要通过使用3 种类型的纳米颗粒标记物来增强。LFIA中的纳米颗粒标记物按材料可分为有色纳米颗粒、发光纳米颗粒以及磁性纳米颗粒等。其中,发光纳米颗粒又包括量子点(quantum dots,QDs)及时间分辨荧光微球(time-resolved fluorescent microspheres,TRFNs)、上转换荧光纳米颗粒(up conversion nanoparticles,UCP)、染料掺杂的荧光纳米颗粒等;有色纳米颗粒包括常规胶体金纳米颗粒(gold nanoparticles,GNPs)、碳纳米颗粒(carbon nanoparticles,CNPs)和胶体硒纳米颗粒(colloidal selenium nanoparticles,SNPs)等[5-6]。表1总结了不同的纳米颗粒在LFIA中的优缺点。
表1 不同的纳米颗粒在LFIA中的优缺点
Table 1 Advantages and disadvantages of different nanoparticles in LFIA
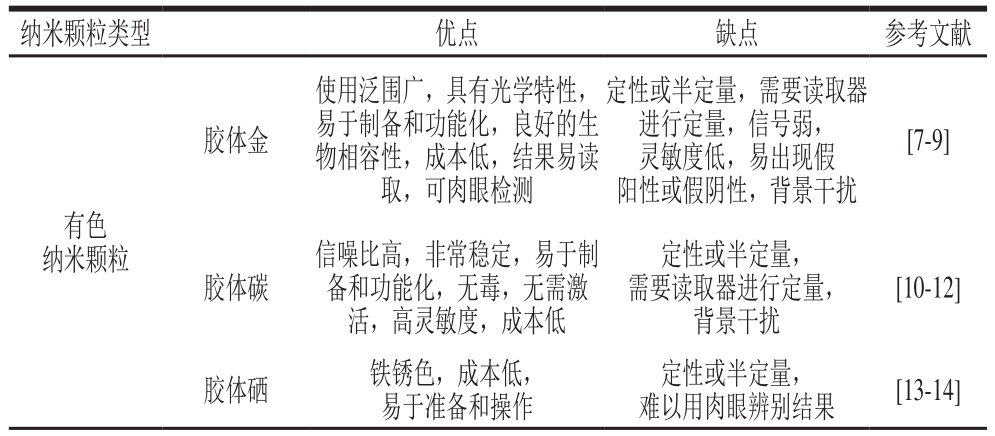
纳米颗粒类型 优点 缺点 参考文献胶体金使用泛围广,具有光学特性,易于制备和功能化,良好的生物相容性,成本低,结果易读取,可肉眼检测定性或半定量,需要读取器进行定量,信号弱,灵敏度低,易出现假阳性或假阴性,背景干扰[7-9]有色纳米颗粒胶体碳信噪比高,非常稳定,易于制备和功能化,无毒,无需激活,高灵敏度,成本低定性或半定量,需要读取器进行定量,背景干扰[10-12]胶体硒 铁锈色,成本低,易于准备和操作定性或半定量,难以用肉眼辨别结果 [13-14]
续表1
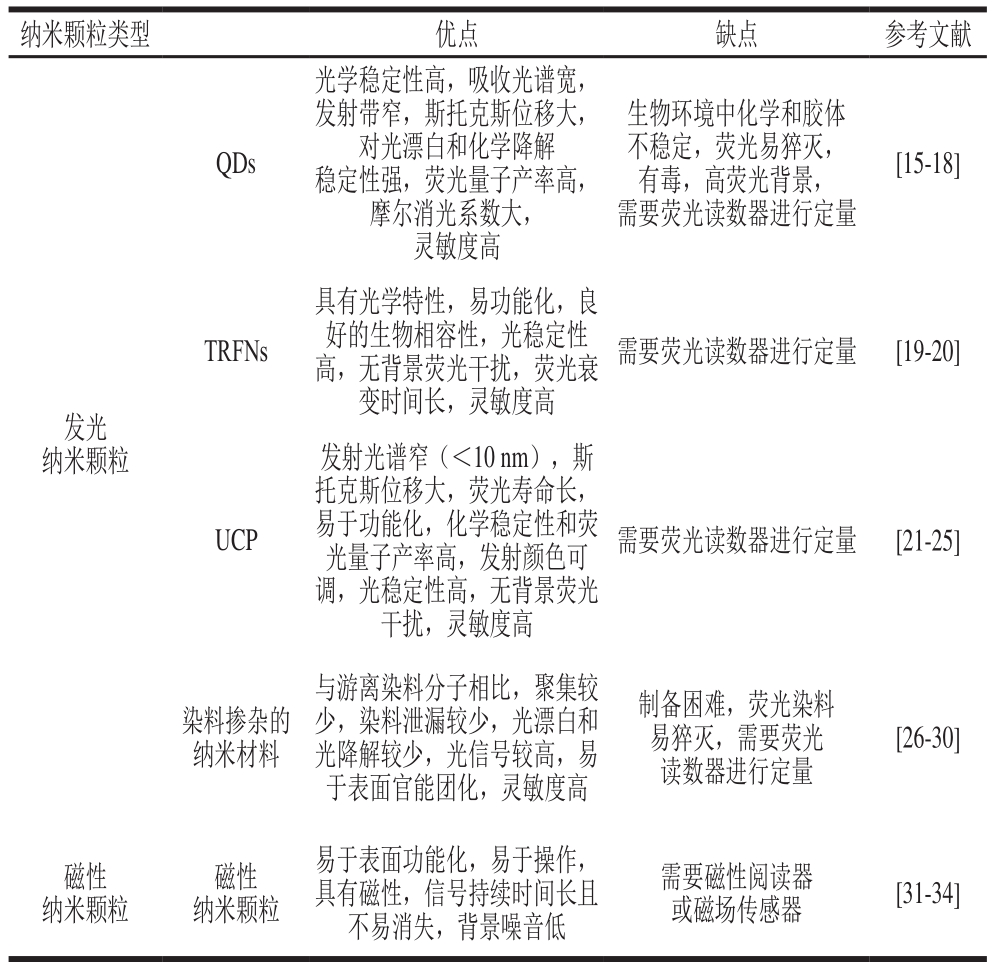
纳米颗粒类型 优点 缺点 参考文献QDs光学稳定性高,吸收光谱宽,发射带窄,斯托克斯位移大,对光漂白和化学降解稳定性强,荧光量子产率高,摩尔消光系数大,灵敏度高生物环境中化学和胶体不稳定,荧光易猝灭,有毒,高荧光背景,需要荧光读数器进行定量[15-18]TRFNs具有光学特性,易功能化,良好的生物相容性,光稳定性高,无背景荧光干扰,荧光衰变时间长,灵敏度高需要荧光读数器进行定量 [19-20]发光纳米颗粒UCP发射光谱窄(<10 nm),斯托克斯位移大,荧光寿命长,易于功能化,化学稳定性和荧光量子产率高,发射颜色可调,光稳定性高,无背景荧光干扰,灵敏度高需要荧光读数器进行定量 [21-25]染料掺杂的纳米材料与游离染料分子相比,聚集较少,染料泄漏较少,光漂白和光降解较少,光信号较高,易于表面官能团化,灵敏度高制备困难,荧光染料易猝灭,需要荧光读数器进行定量[26-30]磁性纳米颗粒磁性纳米颗粒易于表面功能化,易于操作,具有磁性,信号持续时间长且不易消失,背景噪音低需要磁性阅读器或磁场传感器 [31-34]
2.1 有色纳米颗粒
2.1.1 胶体金纳米颗粒
GNPs由氯金酸在还原剂柠檬酸三钠等的作用下聚合成为特定大小的金颗粒,并通过静电作用而形成的一种稳定的胶体状态,直径在l~150 nm,属于多相不均匀体系,颜色呈桔红色到紫红色。胶体金因具有颜色鲜亮、易于制备、生物相容性佳、高度稳定及化学可示踪性及良好光学性状等诸多优点而被广泛用作LFIA标记探针[35]。
2.1.2 碳纳米颗粒
CNPs也称为胶体碳或炭黑,粒径在10~100 nm之间,是一类有色颗粒标记物,可进行定性或半定量检测。作为GNPs的替代标记物,CNPs具有许多优异的性质,例如易制备、高稳定性、无毒性、易于缀合及不需活化等[36]。
2.1.3 胶体硒纳米颗粒
SNPs也称为胶体硒,是另一种应用于免疫检测技术领域的纳米标记物。与胶体金类似,同样是通过调整反应条件获得不同纳米级别的硒颗粒,具有表面效应和小尺寸效应。另外,它还具有成本低、与蛋白标记简单、标记后不易聚沉以及便于调整粒径范围等优点[37]。
2.2 发光纳米颗粒
2.2.1 量子点
QDs是一种荧光半导体纳米晶体,一般为球形或类球形,粒径通常在2~20 nm之间。常见的QDs由IV、II~VI、IV~VI或III~V族元素组成,具体包括硅QDs、锗QDs、硫化镉QDs、硒化镉QDs、碲化镉QDs、硒化锌QDs、硫化铅QDs、硒化铅QDs、磷化铟QDs和砷化铟QDs等[38]。QDs具有独特的光学性质,如激发光谱宽且连续分布,而发射光谱窄而对称,光化学稳定性强以及荧光寿命长等优越的荧光特性,被认为是纸基分析中最有潜力的生物标记物。
2.2.2 上转换荧光纳米颗粒
UCP是一种由主基质(氟化物等)、吸收子(Er、Yb、Sm等稀土离子)及发射子(Tm、Ho、Tb等稀土离子)组成的荧光物质,是一类新近开发的荧光探针,与普通的下转换纳米材料相比,UCP是用低能量的近红外光激发,发射高能量的可见光;因此,对生物组织伤害小且穿透能力强,是理想的荧光纳米材料[39-40]。
2.2.3 染料掺杂的纳米颗粒
2.2.3.1 染料掺杂的二氧化硅纳米颗粒
二氧化硅纳米颗粒的尺寸和粒度分布均一、成本低,具有良好的生物相容性和丰富的表面基团可以进行修饰。与QDs和UCP不同,二氧化硅纳米颗粒自身虽不能发光,但可将功能性物质包裹或掺杂到二氧化硅纳米颗粒中,如荧光染料、金纳米颗粒、QDs、稀土发光材料等,从而形成发光复合纳米材料。鉴于以上优点,二氧化硅纳米颗粒被广泛应用于生物检测领域[41]。目前,研究人员开发了掺杂有镧系元素螯合物的二氧化硅纳米颗粒,以增强二氧化硅纳米颗粒的荧光性能并提高检测灵敏度。TRFNs指添加了具有荧光特性物质(以镧系元素为代表)的二氧化硅纳米颗粒[42]。
2.2.3.2 染料掺杂的聚苯乙烯纳米颗粒
聚苯乙烯纳米颗粒是另一类用于掺杂染料分子的纳米材料。与染料掺杂的二氧化硅纳米颗粒相似,染料掺杂的聚苯乙烯纳米颗粒可以提供比游离染料分子更高的荧光信号,从而提高膜基LFIA的分析性能[43]。
2.3 磁性纳米颗粒
磁性纳米颗粒(magnetic nanoparticles,MNPs)指尺寸为纳米级的磁性材料(以Fe3O4为代表),具有良好的磁导向性和生物相容性,易于制备,可与抗体耦合形成免疫磁珠[44]。
3 纳米颗粒在LFIA中的功能及应用
LOD、特异性和检测时间是评估LFIA技术的主要标准。纳米材料的引入为LFIA技术实现高灵敏、高特异性和快速检测奠定了良好基础[45-46]。
3.1 提高检测灵敏度
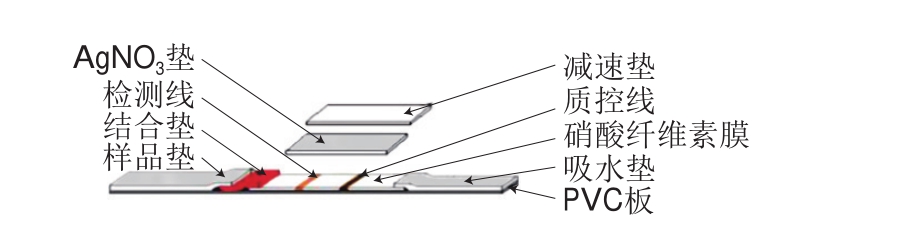
在传统的免疫层析(immunochromatographic assay,ICA)技术中,胶体金被用作标记物,然而胶体金较低的光学信号以及较少的抗体吸附量限制了ICA技术灵敏度的提高,因此标记材料的选择对提高ICA技术的灵敏度具有重要意义。目前,已报道了大量提高LFIA灵敏度的研究,一种策略是利用许多有色纳米颗粒作为标记物,以提高测定灵敏度,如碳纳米管和TRFNs、QDs和UCP[47]。在TRFNs中,每个荧光微球可包裹成千上万个荧光分子,荧光标记率高,有效提高了分析灵敏度。例如,Zhang Yong等[48]对盐酸克仑特罗进行定量检测,系统地比较了TRFNs-LFIA和GNPs-LFIA,LOD分别为16、68 pg/mL,TRFNs-ICA的灵敏度优于胶体金免疫层析(colloidal gold-ICA,CG-ICA),但TRFNs-LFIA的基质效应高。金纳米花(gold nano flower,AuNF)的多分支结构和大的比表面积使其具有强光学信号和亲和力,因此AuNF制备的ICA试纸条灵敏度更高。如Zhang Wenjing等[49]合成具有5 种不同粒径大小(33、47、79、152、195 nm)的AuNF,并将它们作为标记物用于人绒毛膜促性腺激素检测,直径为47~79 nm的中型AuNF显示出的灵敏度最高(9 mIU/mL),比传统的基于CNPs的LFIA技术的LOD低10 倍。另外,研究人员开发了银增强、金增强、酶增强、双标记等策略以实现高灵敏度的定量测定,对实现LFIA超灵敏检测具有重要意义。虽然通过银或者金来放大金标记信号是有效的增敏途径,但仍受到银溶液制备以及洗涤的限制。为克服这些问题,研究人员对传统LFIA进行改进。例如Yang Wei等[50]改进了银放大金标信号的夹心LFIA以检测相思子毒素,与传统的LFIA相比,增加了AgNO3垫和减速垫(还原剂垫)(图1),LOD为0.1 ng/mL,灵敏度增加了100 倍。目前,研究人员利用GNPs光热效应性质,用特定波长的激光照射激发GNPs的光热效应进而使温度升高,利用此特性,可将光热效应应用于胶体金试纸条方法,使灵敏度明显提高。刘静静[51]利用纳米材料光热效应检测大肠杆菌,灵敏度提高了10 倍。
对于荧光纳米颗粒,尽管可以通过多种策略实现高灵敏度,但荧光信号易于猝灭并且遭受光漂白的问题,有可能导致灵敏度降低并且不适合大规模生产和长期保存。在过去的10 年中,超顺磁性纳米颗粒(superparamagnetic nanoparticles,SMNPs)作为一种新型标记材料引起了极大的关注,通过检测NC检测带表面的磁信号,取代传统的光学信号,极大地提高了敏感性和准确性。Wang Yanyan等[44]使用不同尺寸和磁铁矿含量的SMNPs作为标签研究定量LFIA技术并利用巨磁传感器检测,灵敏度提高10~1 000 倍。另外,Razo等[52]将GNPs和SMNPs联合用于测流ICA试纸条,实现信号双重增强,检测马铃薯病毒X(图2),灵敏度明显提高,解决了因SMNPs的高荧光猝灭性质和磁性荧光探针无法进行定量检测的问题。
目前,LFIA技术除了改善标记材料来提高灵敏度以外,寻找识别力更强的物质替代抗体与抗原特异性反应、设计新型试纸条来提高ICA试纸条的检测灵敏度已成为新的趋势。另外,在传统的光学LFIA技术中,主要使用反射光或荧光的阅读器进行定量测定。膜的厚度为几百微米[34],捕获的分析物在膜表面移动,同时会向膜内渗透。然而读取器对膜进行扫描时仅检测到了膜表面(约10 μm)分析物的信号,约90%的分析物未被检测到,这也是试纸条灵敏度低的原因之一。因此除了改善标记物以外,如何更准确地读取试纸条信息,也受到越来越多的关注。研究人员开发基于智能手机的成像系统,用于定量检测,将智能成像引入LFIA中准确性明显提高[53]。
3.2 实现高通量检测
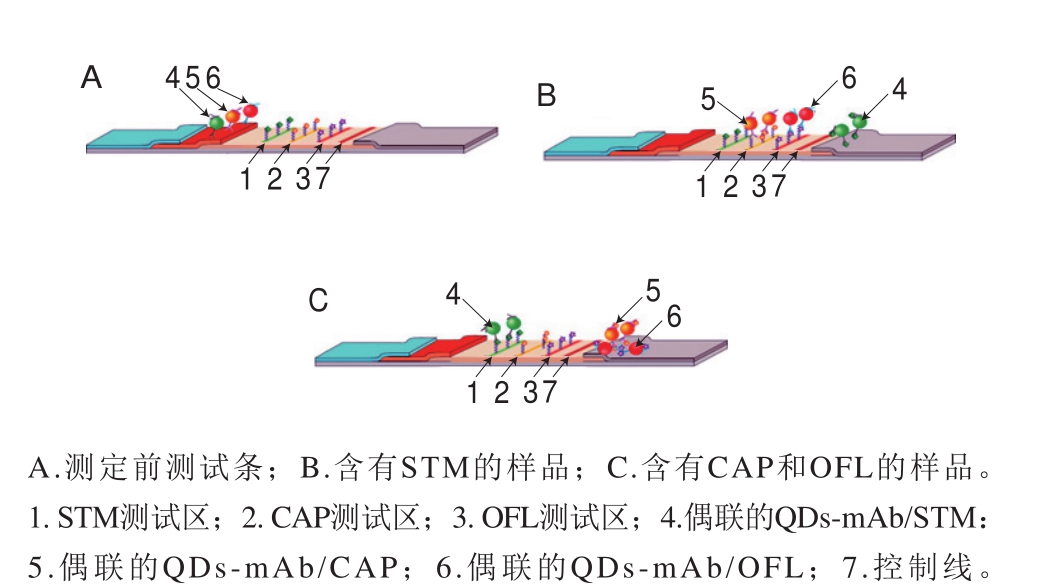
传统LFIA实现多元检测主要是在单个LFIA试纸条中设计多条检测线。Taranova等[54]通过使用多色QDs对牛乳中的氧氟沙星(ofloxacin,OFL)、氯霉素(chloramphenicol,CAP)和链霉素(streptomycin,STM)同时检测,设计了一种“交通灯”的竞争性LFIA技术(图3)。该设计使用在625、585 nm和525 nm波长处具有发射峰的3 种水溶性QDs,分别产生红色、黄色和绿色的3 个测试区。在“交通灯”分析中,OFL、CAP和STM的LOD分别达到0.3、0.12、0.2 ng/mL,比酶联免疫吸附检测(enzyme linked immune sorbent assay,ELISA)的LOD低80~200 倍。利用新型纳米材料(QDs、UCP)发射颜色可调性,可以实现在只有一条检测线和质控线情况下进行多元检测。Wang Chunying等[55]开发了一种基于多色QDs的三明治式LFIA技术(图4),同时检测甲胎蛋白(alpha-fetoprotein,AFP)和癌胚抗原(carcinoembryonic antigen,CEA),LOD分别为3 ng/mL和2 ng/mL。此外,为避免多元检测试纸条交叉问题。Hong Wenyan等[56]开发基于UCP双抗夹心模式的十通道试纸盘检测系统,同时检测经鼠疫杆菌感染的动物及人血清样品中的10 种抗体靶标,检测得到F1抗体阳性率100%。该装置的分析性能与常规ELISA相当。
多元检测也是ICA技术发展的方向,且日益趋于成熟,即在同一条膜上同时检测多种物质,以提高检测效率,降低检测成本。多元检测尤其对有联检意义的多个指标的检测有很大的应用价值。
3.3 通过可修饰性降低假阳性
表2 不同纳米颗粒的LFIA试纸条在生物检测中的应用
Table 2 Application of nanoparticle-based lateral flow immunochromatographic test strips in food safety testing

纳米颗粒 目标分析物 LOD 检测时间 参考文献克伦特罗 220 pg/mL 10 min [13]大肠杆菌O157:H7 5×103 CFU/mL 20 min [60]微囊藻毒素 5 ng/mL [61]霍乱弧菌O139 104 CFU/mL [62]铅离子 0.19 ng/mL 15 min [63]汞0.001 5 pg/mL 10 min [64]黄曲霉毒素B1 2.0 ng/mL 10 min [65]赭曲霉毒素A 0.9 μg/L 20 min [66]短链霉素 20 ng/mL 10 min [67]无乳链球菌 1.5×105 CFU 15 min [68]纳米花束 黄曲霉毒素B2 0.32 pg/mL 15 min [12]大肠杆菌O157:H7 103 CFU/mL 15 min [69]胶体金胶体碳膨大剂 33.4 μg/kg [70]产志贺毒素大肠埃希氏菌 104~105 CFU/mL [15]甲萘威 1.5 ng/mL 10 min [71]噻菌灵 0.08 ng/mL 10 min [72]氯霉素 0.2 ng/mL 20 min [53]赭曲霉毒素A 1.9 ng/mL 10 min [74]QDs大肠埃希氏菌、单增李斯特菌和肠道沙门氏菌 300~600 cells/test [75]伏马菌素 2.8 μg/L 22 min [76]副溶血性弧菌 4×102 CFU/mL [77]金黄色葡萄球菌 108 CFU/mL [78]有机磷 l.0 ng/mL 10 min [79]克伦特罗 0.5 μg/L [80]莱克多巴胺 3 ng/mL 10 min [81]布鲁氏菌 5×106 CFU/mL 15 min [82]黄曲霉毒素B1 0.03 ng/mL [83]相思子毒素 0.1 μg/kg 20 min [84]磺胺喹恶啉 1 μg/L 15 min [85]NaYF4:Yb3+、Er3+掺杂的二氧化硅纳米颗粒UCP鼠疫耶尔森氏菌 104 CFU/mL 15 min [86]鳗弧菌 102 CFU/mL 15 min [35]Eu3+-BHHCT掺杂的二氧化硅纳米颗粒 克伦特罗 0.037 ng/mL 10 min [87]Ru(phen)32+掺杂的二氧化硅纳米颗粒恩诺沙星 0.02 ng/mL 20 min [88]沙丁胺醇 0.43 ng/mL 15 min [89]大肠杆菌O157:H7 104 CFU/mL 10 min [90]黄曲霉毒素B1 2.5 μg/L 10 min [91]伏马菌素B1 0.12 ng/mL 20 min [92]Eu3+螯合物掺杂的聚苯乙烯纳米颗粒FITC掺杂的聚苯乙烯纳米颗粒赭曲霉毒素A 1.0 μg/kg 8 min [93]T-2毒素 0.09 ng/g 12 min [47]磁性纳米颗粒炭疽芽孢杆菌孢子 400 个孢子 30 min [94]炭疽芽孢杆菌孢子 500~700 个孢子 20 min [95]阪崎肠杆菌 102 CFU/mL 2 h [96]单增李斯特菌 104 CFU/mL [97]
诸多影响因素,如样品、LFIA本身的设计缺陷或操作不当,使得ICA技术在实际检测过程中仍会出现假阳性。产生假阳性的主要原因是由于检测环境、样品基质等的干扰,容易破坏胶体金与蛋白质大分子间的静电结合力,导致胶体金标记率低、产生裸金,蛋白与胶体金颗粒非特异性结合。一般情况下,在LFIA中为减少假阳性概率的出现,通常会选择惰性蛋白对硝酸纤维素膜、纳米标记物进行封闭。近年来研究人员利用修饰性纳米颗粒,避免蛋白质与纳米颗粒非特异性结合,从而降低假阳性率。TRFNs除提高灵敏度外,由于其表面修饰有合适密度的羧基,用于与蛋白或抗体的共价偶联,提高标记率,有效避免假阳性,已经逐渐取代第一代胶体金、彩色乳胶和第二代荧光微球技术[57]。罗凯[58]研究胶体金、QDs、荧光微球和TRFNs4 种标记物用于测定大肠杆菌O157:H7。由于TRFNs的修饰性强,有效避免了非特异性结合,使基于铕荧光微球的时间分辨ICA检测方法灵敏度最高,为5.0×102 CFU/mL,假阳性率较低,且对牛乳基质的耐受性能更好。目前,除了利用可修饰纳米颗粒降低假阳性概率,对抗体进行修饰、增强抗体的特异性结合能力也成为降低假阳性概率的一个有效途径[59]。
由于各种新型纳米材料标记物的不断研发与优化分析,使得与之相关的检测技术广泛运用于医药、食品安全和环境监测等方面。表2总结了不同纳米颗粒的LFIA试纸条在各领域中的应用。
4 结 语
LFIA已经历经几十年发展而较为成熟,已实现商品化和规模化应用,但该系统在检出限、灵敏度、定量测定和高通量检测方面仍需改进。纳米颗粒具有独特的性质,如比表面积大、表面反应活性高。固有信号放大、光学分析谱和控制处理能力等,能够改善LFIA技术分析性能。本文简要总结了基于纳米颗粒标记物的LFIA技术,以及纳米颗粒对试纸条分析性能的改善作用。表明基于新型纳米颗粒可以提高LFIA技术的灵敏度和稳定性。同时,基于纳米颗粒的多重检测层析技术已被开发建立。此外,纳米颗粒也可用作电化学或光学传导系统的修饰剂。
尽管纳米颗粒对LFIA技术性能有很大的改善,但在实际应用中仍存在很多问题。目前,主要面临的问题及发展的趋势简述如下:1)抗体对侧流技术的影响。例如,捕获和检测抗体应该具有高纯度以避免基质干扰。此外,由于空间位阻、强亲水性和纳米颗粒的疏水性,抗体对靶分析物的亲和力或活性会显著降低,因此纳米颗粒缀合抗体应适当被优化,以保持抗体-纳米颗粒缀合物的高活性和稳定性[98]。同时改进纳米颗粒与抗体的缀合过程,以保持抗体-纳米颗粒缀合物的高活性。抗体-纳米颗粒缀合物的长期稳定性和可用性在灵敏度和选择性方面对LFIA技术起着重要作用。2)硝酸纤维素膜的选择。LFIA技术中膜材料的作用是确保每个组件的适用性和兼容性,并降低假阴性或假阳性信号。Lee等[99]研究LFIA技术中选择合适的膜用于敏感检测低分子质量化合物的重要性。3)检测基质的复杂性。由于不同食品样品的电解质浓度和pH值往往会有很大差异,纳米颗粒标记物在不同检测基质中的稳定性和单分散性需进一步提高和改善[100]。对于荧光纳米颗粒样品基质有可能带来高的背景干扰,严重影响灵敏度,如何消除样品基质的干扰有待进一步研究。随着纳米技术与分子生物学技术的发展,各种纳米颗粒作为新型标志物在改进LFIA技术方面显示出巨大的潜力,将广泛用于临床诊断、食品安全性和环境监测等领域。
参考文献:
[1] OMIDFAR K, KHORSAND F, DARZIANI A M. New analytical applications of gold nanoparticles as label in antibody based sensors[J].Biosensors & Bioelectronics, 2013, 43(1): 336-347. DOI:10.1016/j.bios.2012.12.045.
[2] 马兰, 王淑娟, 曾海娟, 等. 侧流层析技术研究进展[J]. 食品科学,2018, 39(15): 343-352. DOI:10.7506/spkx1002-6630-201815048.
[3] WANG X, NIESSNER R, TANG D, et al. Nanoparticle-based immunosensors and immunoassays for aflatoxins[J]. Analytica Chimica Acta, 2016, 912: 10-23. DOI:10.1016/j.aca.2016.01.048.
[4] 王广凤, 朱艳红, 陈玲, 等. 功能性纳米材料在电化学免疫传感器中的应用[J]. 分析化学, 2013, 41(4): 608-615. DOI:10.3724/SP.J.1096.2013.20611.
[5] CHUN P. Colloidal gold and other labels for lateral flow immunoassays[M]. New Jersey: Humana Press, 2009: 1-19.DOI:10.1007/978-1-59745-240-3-5.
[6] FAULSTICH K, GRULER R, EBERHARD M, et al. Handheld and portable reader devices for lateral flow immunoassays[M]. New Jersey:Humana Press, 2009: 1-27. DOI:10.1007/978-1-59745-240-3-9.
[7] ELAHI N, KAMALI M, BAGHERSAD M H. Recent biomedical applications of gold nanoparticles: a review[J]. Talanta, 2018, 184:537-556. DOI:10.1016/j.talanta.2018.02.088.
[8] POSTHUMA-TRUMPIE G A, WICHERS J H, KOETS M, et al.Amorphous carbon nanoparticles: a versatile label for rapid diagnostic(immuno) assays[J]. Analytical & Bioanalytical Chemistry, 2012,402(2): 593-600. DOI:10.1007/s00216-011-5340-5.
[9] SAKR T M, KORANY M, KATTI K V. Selenium nanomaterials in biomedicine: an overview of new opportunities in nanomedicine of selenium[J]. Journal of Drug Delivery Science & Technology, 2018,46: 223-233. DOI:10.1016/j.jddst.2018.05.023.
[10] RESCHGENGER U, GRABOLLE M, CAVALIEREJARICOT S, et al.Quantum dots versus organic dyes as fluorescent labels[J]. Nature Methods, 2008, 5(9): 763-775. DOI:10.1038/nmeth.1248.
[11] MENDEZ-GONZALEZ D, LOPEZ-CABARCOS E,RUBIORETAMA J, et al. Sensors and bioassays powered by upconverting materials[J]. Advances in Colloid & Interface Science,2017, 249: 66-87. DOI:10.1016/j.cis.2017.06.003.
[12] LI X, ZHANG F, ZHAO D. Highly efficient lanthanide upconverting nanomaterials: progresses and challenges[J]. Nano Today, 2013, 8(6):643-676. DOI:10.1016/j.nantod.2013.11.003.
[13] 李晓彤. 复合荧光二氧化硅纳米粒子的可控制备及其荧光性能探究[D]. 武汉: 华中农业大学, 2014: 5-20.
[14] WANG Zhizeng, JING Jing, REN Yangguang, et al. Preparation and application of selenium nanoparticles in a lateral flow immunoassay for clenbuterol detection[J]. Materials Letters, 2019, 234: 212-215.DOI:10.1016/j.matlet.2018.09.056.
[15] CHATTERJEE J, HAIK Y, CHEN C J. Modification and characterization of polystyrene-based magnetic microspheres and comparison with albumin-based magnetic microspheres[J]. Journal of Magnetism & Magnetic Materials, 2001, 225(1): 21-29. DOI:10.1016/S0304-8853(00)01223-3.
[16] QU H, ZHANG Y, QU B, et al. Rapid lateral-flow immunoassay for the quantum dot-based detection of puerarin[J]. Biosensors and Bioelectronics, 2016, 81: 358-362. DOI:10.1016/j.bios.2016.03.008.
[17] XIAO M, FU Q Q, SHEN H C, et al. A turn-on competitive immunochromatographic strips integrated with quantum dots and gold nano-stars for cadmium ion detection[J]. Talanta, 2017, 178: 644-649.DOI:10.1016/j.talanta.2017.10.002.
[18] XIE Y, ZHANG L, YANG X, et al. Development of a quantum dot-based immunochromatography test strip for rapid screening of oxytetracycline and 4-epi-oxytetracycline in edible animal tissues[J].Food Additives & Contaminants, 2017, 34(3): 371-378. DOI:10.1080/19440049.2016.1277038.
[19] XU H, CHEN J, BIRRENKOTT J, et al. Gold-nanoparticle-decorated silica nanorods for sensitive visual detection of proteins[J]. Analytical Chemistry, 2014, 86(15): 7351-7359. DOI:10.1021/ac502249f.
[20] NOGUERA P, POSTHUMA-TRUMPIE G A, VAN TUIL M, et al.Carbon nanoparticles in lateral flow methods to detect genes encoding virulence factors of Shiga toxin-producing Escherichia coli[J].Analytical & Bioanalytical Chemistry, 2011, 399(2): 831-838.DOI:10.1007/s00216-010-4334-z.
[21] NOGUERA P S, POSTHUMA-TRUMPIE G A, VAN TUIL M, et al.Carbon nanoparticles as detection labels in antibody microarrays.detection of genes encoding virulence factors in Shiga toxin-producing Escherichia coli[J]. Analytical Chemistry, 2011, 83(22): 8531-8536.DOI:10.1021/ac201823v.
[22] GORDON J, MICHEL G. Analytical sensitivity limits for lateral flow immunoassays[J]. Clinical Chemistry, 2008, 54(7): 1250-1251.DOI:10.1373/clinchem.2007.102491.
[23] GNACH A, BEDNARKIEWICZ A. Lanthanide-doped up-converting nanoparticles: merits and challenges[J]. Nano Today, 2012, 7(6): 532-563. DOI:10.1016/j.nantod.2012.10.006.
[24] SHIRSAT S, KADAM A, NAUSHAD M, et al. Selenium nanostructures: microbial synthesis and applications[J]. RSC Advances, 2015, 5: 92799-92811. DOI:10.1039/c5ra17921a.
[25] SHAO Q Y, LI X S, HUA P Y, et al. Enhancing the upconversion luminescence and photothermal conversion properties of ~800 nm excitable core/shell nanoparticles by dye molecule sensitization[J].Journal of Colloid and Interface Science, 2017, 486: 121-127.DOI:10.1016/j.jcis.2016.09.067.
[26] NÄREOJA T, VEHNIÄINEN M, LAMMINMÄKI U, et al. Study on nonspecificity of an immuoassay using Eu-doped polystyrene nanoparticle labels[J]. Journal of Immunological Methods, 2009,345(1): 80-89. DOI:10.1016/j.jim.2009.04.008.
[27] LIU M, LIU Z Y, LÜ Q, et al. Functionalized fluorescein-doped SiO2 nanoparticles for immunochromatographic assay[J]. Chinese Journal of Chemistry, 2005, 23(7): 875-880. DOI:10.1002/cjoc.200590875.
[28] ZHOU Jie, ZHU Kui, XU Fei, et al. Development of a Microspherebased fluorescence immunochromatographic assay for monitoring lincomycin in milk, honey, beef, and swine urine[J]. Journal of Agricultural and Food Chemistry, 2014, 62(49): 12061-12066.DOI:10.1021/jf5029416.
[29] NHUNG T H, NGHIEM T H L, THI T D V, et al. Dye-doped silicabased nanoparticles for bioapplications[J]. Advances in Natural Sciences Nanoscience & Nanotechnology, 2013, 4(4): 043001.DOI:10.1088/2043-6262/4/4/043001.
[30] RICCÒ R, NIZZERO S, PENNA E, et al. Ultra-small dye-doped silica nanoparticles via modified sol-gel technique[J]. Journal of Nanoparticle Research, 2018, 20(5): 117. DOI:10.1007/s11051-018-4227-1.
[31] ZHENG C, WANG X, LU Y, et al. Rapid detection of fish major allergen parvalbumin using superparamagnetic nanoparticle-based lateral flow immunoassay[J]. Food Control, 2010, 26(2): 446-452.DOI:10.1016/j.foodcont.2012.01.040.
[32] PATERSON A S, RAJA B, GARVEY G, et al. Persistent luminescence strontium aluminate nanoparticlesas reporters in lateral flow assays[J]. Analytical Chemistry, 2014, 86(19): 9481-9488.DOI:10.1021/ac5012624.
[33] WANG F, BANERJEE D, LIU Y, et al. Upconversion nanoparticles in biological labeling, imaging, and therapy[J]. Analyst, 2010, 135(8):1839-1854. DOI:10.1039/C0AN00144A.
[34] HAN G M, LI H, HUANG X X, et al. Simple synthesis of carboxyl-functionalized upconversion nanoparticles for biosensing and bioimaging applications[J]. Talanta, 2016, 147: 207-212.DOI:10.1016/j.talanta.2015.09.059.
[35] ZHAO P, WU Y, ZHU Y, et al. Upconversion fluorescent strip sensor for rapid determination of Vibrio anguillarum[J]. Nanoscale, 2014,6(7): 3804-3809. DOI:10.1039/c3nr06549a.
[36] ZHANG X, YU X, WEN K, et al. Multiplex lateral flow immunoassays based on amorphous carbon nanoparticles for detecting three Fusantium mycotoxins in maize[J]. Journal of Agricultural and Food Chemistry, 2017, 65(36): 8063-8071. DOI:10.1021/acs.jafc.7b02827.
[37] WANG Z, ZHI D, YANG Z, et al. Lateral flow test strip based on colloidal selenium immunoassay for rapid detection of melamine in milk, milk powder, and animal feed[J]. International Journal of Nanomedicine, 2014, 2014: 1699-1707. DOI:10.2147/IJN.S58942.
[38] REN M, XU H, HUANG X, et al. Immunochromatographic assay for ultrasensitive detectionof aflatoxin B1 in maize by highly luminescent quantum dot beads[J]. ACS Applied Materials & Interfaces, 2014,6(16): 14215-14222. DOI:10.1021/am503517s.
[39] LEI L, ZHOU Y, HAN Y, et al. Rapid detection of serum procalcitonin by immunochromatograghy technology based on freeze-dried upconvertion nanoparticles/ antibody conjugates[J]. Chinese Journal of Chemistry, 2017, 35(12): 1861-1868. DOI:10.1002/cjoc.201700354.
[40] LIAN Y, DING L J, ZHANG W, et al. Synthesis of highly stable cyanine-dye-doped silica nanoparticle for bological aplications[J].Methods and Applications in Fluorescence, 2018, 6(3): 034002.DOI:10.1088/2050-6120/aab930.
[41] RYU Y, JIN Z, KANG M S, et al. Increase in the detection sensitivity of a lateral flow assay for a cardiac marker by oriented immobilization of antibody[J]. BioChip Journal, 2011, 5(3): 193-198. DOI:10.1007/s13206-011-5301-2.
[42] SERRATE D, DE TERESA J M, MARQUINA C, et al. Quantitative biomolecular sensing station based on magnetoresistive patterned arrays[J]. Biosensors & Bioelectronics, 2012, 35(1): 206-212.DOI:10.1016/j.bios.2012.02.048.
[43] ZHANG D K, WANG Y P, MA D G. Random lasing emission from a red fluorescent dye doped polystyrene film containing dispersed polystyrene nanoparticles[J]. Applied Physics Letters, 2007, 91(9):91115. DOI:10.1063/1.2778550.
[44] WANG Yanyan, XU Hong, WEI Meng, et al. Study of superparamagnetic nanoparticles as labels in the quantitative lateral flow immunoassay[J]. Materials Science & Engineering C, 2009,29(3): 714-718. DOI:10.1016/j.msec.2009.01.011.
[45] LI Yue, WANG Zhongxing, SUN Li, et al. Nanoparticle-based sensors for food contaminant[J]. Trends in Analytical Chemistry, 2019, 113:74-83. DOI:10.1016/j.trac.2019.01.012.
[46] OMIDFAR K, KIA S, LARIJANI B. Development of a colloidal gold-based immunochromatographic test strip for screening of microalbuminuria[J]. Hybridoma, 2011, 30(2): 117-124. DOI:10.1089/hyb.2010.0090.
[47] ZVEREVA E A, ZHERDEV A V, XU C, et al. Highly sensitive immunochromatographic assay for qualitative and quantitative control of beta-agonist salbutamol and its structural analogs in foods[J]. Food Control, 2017, 86: 50-58. DOI:10.1016/j.foodcont.2017.11.003.
[48] ZHANG Yong, PENG Juan, GUO Ping, et al. Matrix effect of swine urine on time-resolved fluorescent nanobeads and colloidal gold immunochromatographic assay[J]. Food & Agricultural Immunology,2018, 29(1): 711-721. DOI:10.1080/09540105.2018.1439456.
[49] ZHANGA Wenjing, DUAN Hong, CHEN Rui, et al. Effect of different-sized gold nanoflowers on the detection performance of immunochromatographic assay for human chorionic gonadotropin detection[J]. Talanta, 2019, 194: 604-610. DOI:10.1016/j.talanta.2018.10.080.
[50] YANG Wei, LI Xiaobing, LIU Guowen, et al. A colloidal gold probebased silver enhancement immunochromatographic assay for the rapid detection of abrin-a[J]. Biosensors & Bioelectronics, 2011, 26(8):3710-3713.
[51] 刘静静. 基于纳米材料光热效应的食源性致病菌试纸条检测方法研究[D]. 济南: 山东师范大学, 2017: 10-25.
[52] RAZO S C, PANFEROV V G, SAFENKOVA I V, et al. Doubleenhanced lateral flow immunoassay for potato virus X based on a combination of magnetic and gold nanoparticles[J]. Analytica Chimica Acta, 2018, 1007: 50-60. DOI:10.1016/j.aca.2017.12.023.
[53] MAK W C, BENI V, TURNER A P F. Lateral-flow technology: from visual to instrumental[J]. Trends in Analytical Chemistry, 2016, 79:297-305. DOI:10.1016/j.trac.2015.10.017.
[54] TARANOVA N A, BERLINA A N, ZHERDEV A V, et al. ‘Traffic light’immunochromatographic test based on multicolor quantum dots for the simultaneous detection of several antibiotics in milk[J]. Biosensors &Bioelectronics, 2015, 63(2): 255-261. DOI:10.1016/j.bios.2014.07.049.
[55] WANG Chunying, HOU Fei, MA Yisai. Simultaneous quantitative detection of multiple tumor markers with a rapid and sensitive multicolor quantum dots based immunochromatographic test strip[J].Biosensors & Bioelectronics, 2015, 68: 156-162. DOI:10.1016/j.bios.2014.12.051.
[56] HONG Wenyan, HUANG Lihua, WANG Haoran, et al. Development of an up-converting phosphor technology-based 10-channel lateral flow assay for profiling antibodies against Yersinia pestis[J]. Journal of Microbiological Methods, 2010, 83: 133-140. DOI:10.1016/j.mimet.2010.08.005.
[57] HU L M, LUO K, XIA J, et al. Advantages of time-resolved fluorescent nanobeads compared with fluorescent submicrospheres,quantum dots, and colloidal gold as label in lateral flow assays for detection of ractopamine[J]. Biosensors & Bioelectronics, 2017, 91:95-103. DOI:10.1016/j.bios.2016.12.030.
[58] 罗凯. 盐酸克伦特罗和大肠杆菌O157:H7免疫层析检测方法的研究[D].南昌: 南昌大学, 2017: 6-20.
[59] LOU D, FAN L, CUI Y, et al. Fluorescent nanoprobes with oriented modified antibodies to improve lateral flow immunoassay of cardiac troponin I[J]. Analytical Chemistry, 2018, 90(11): 6502-6508.DOI:10.1021/acs.analchem.7b05410.
[60] ZHAO X H, HE X W, LI W M, et al. Development and evaluation of colloidal gold immunochromatographic strip for detection of Escherichia coli O157[J]. African Journal of Microbiology Research,2010, 4(9): 663-670.
[61] LIU B H, TSAO Z J, WANG J J, et al. Development of a monoclonal antibody against ochratoxin A and its application in enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip[J]. Analytical Chemistry, 2008, 80(18): 7029-7035. DOI:10.1021/ac800951p.
[62] PENGSUK C, CHAIVISUTHANGKURA P, LONGYANT S, et al.Development and evaluation of a highly sensitive immunochromatographic strip test using gold nanoparticle for direct detection of Vibrio cholerae O139 in seafood samples[J]. Biosensors and Bioelectronics, 2013, 42:229-235. DOI:10.1016/j.bios.2012.10.011.
[63] KUANG H, XING C, HAO C, et al. Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor[J]. Sensors,2013, 13(4): 4214-4224. DOI:10.3390/s13040421.
[64] ZHU M, WANG Y, DENG Y, et al. Ultrasensitive detection of mercury with a novel one-step signal amplified lateral flow strip based on gold nanoparticle-labeled ssDNA recognition and enhancement probes[J]. Biosensors & Bioelectronics, 2014, 61(6): 14-20.DOI:10.1016/j.bios.2014.04.049.
[65] LIU B H, HSU Y T, LU C C, et al. Detecting aflatoxin B1 in foods and feeds by using sensitive rapid enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip[J]. Food Control,2013, 30(1): 184-189. DOI:10.1016/j.foodcont.2012.07.008.
[66] ANFOSSI L, DI NARDO F, GIOVANNOLI C, et al. Increased sensitivity of lateral flow immunoassay for ochratoxin A through silver enhancement[J]. Analytical & Bioanalytical Chemistry, 2013, 405(30):9859-9867. DOI:10.1007/s00216-013-7428-6.
[67] ZHOU Y, PAN F G, LI Y S, et al. Colloidal gold probe-based immunochromatographic assay for the rapid detection of brevetoxins in fishery product samples[J]. Biosensors & Bioelectronics, 2009,24(8): 2744-2747. DOI:10.1016/j.bios.2009.01.034.
[68] WU W D, LI M, CHEN M, et al. Development of a colloidal gold immunochromatographic strip for rapid detection of Streptococcus agalactiae in tilapia[J]. Biosensors & Bioelectronics, 2016, 91: 66-69.DOI:10.1016/j.bios.2016.11.038.
[69] ZHANG L, HUANG Y, WANG J, et al. Hierarchical flowerlike gold nanoparticles labeled immunochromatography test strip for highly sensitive detection of Escherichia coli O157:H7[J]. Langmuir, 2015,31(19): 5537-5544. DOI:10.1021/acs.langmuir.5b00592.
[70] CELIA S P, JAN W, ANTONIO A S, et al. Development of an immunochromatographic assay based on carbon nanoparticles for the determination of the phytoregulator forchlorfenuron[J].Biosensors & Bioelectronics, 2013, 42(1): 170-176. DOI:10.1016/j.bios.2012.11.001.
[71] BARBORA H, MARTINA B, LADISLAV F, et al. Development of colloidal carbon-based immunochromatographic strip for rapid detection of carbaryl in fruit juices[J]. European Food Research &Technology, 2010, 231(3): 467-473. DOI:10.1007/s00217-010-1301-z.
[72] BLAZKOVÁ M, RAUCH P, FUKAL L. Strip-based immunoassay for rapid detection of thiabendazole[J]. Biosensors & Bioelectronics,2010, 25(9): 2122-2128. DOI:10.1016/j.bios.2010.02.011.
[73] BERLINA A N, TARANOVA N A, ZHERDEV A V, et al. Quantum dot-based lateral flow immunoassay for detection of chloramphenicol in milk[J]. Analytical & Bioanalytical Chemistry, 2013, 405(14):4997-5000. DOI:10.1007/s00216-013-6876-3.
[74] WANG L, CHEN W, MA W, et al. Fluorescent strip sensor for rapid determination of toxins[J]. Chemical Communications, 2011, 47:1574-1576. DOI:10.1039/c0cc04032k.
[75] BRUNO J G. Application of DNA aptamers and quantum dots to lateral flow test strips for detection of foodborne pathogens with improved sensitivity versus colloidal gold[J]. Pathogens, 2014, 3(2):341-355. DOI:10.3390/pathogens3020341.
[76] NARDO F D, ANFOSSI L, GIOVANNOLI C, et al. A fluorescent immunochromatographic strip test using quantum dots for fumonisins detection[J]. Talanta, 2016, 150: 463-468. DOI:10.1016/j.talanta.2015.12.072.
[77] 许海波, 魏华, 王报贵. 利用双标记PCR产物的量子点检测副溶血性弧菌[J]. 食品工业, 2015, 36(6): 283-287.
[78] CHEN X, GAN M, XU H, et al. Development of a rapid and sensitive quantum dot-based immunochromatographic strip by double labeling PCR products for detection of Staphylococcus aureus in food[J]. Food Control, 2014, 46: 225-232. DOI:10.1016/j.foodcont.2014.04.044.
[79] ZOU Z, DU D, WANG J, et al. Quantum dot-based immunochromatographic fluorescent biosensor for biomonitoring trichloropyridinol, a biomarker of exposure to chlorpyrifos[J].Analytical Chemistry, 2010, 82(12): 5125-5133. DOI:10.1021/ac100260m.
[80] 张金艳, 熊艳, 廖且根, 等. CdTe量子点免疫层析试纸条检测克伦特罗[J]. 食品科学, 2015, 36(22): 161-164. DOI:10.7506/spkx1002-6630-201522030.
[81] 张国华, 赖卫华, 熊勇华, 等. 量子点标记免疫层析试纸条快速检测莱克多巴胺的研究[J]. 食品科学, 2009, 30(12): 254-257.
[82] QU Q, ZHU Z W, WANG Y F, et al. Rapid and quantitative detection of Brucella by up-converting phosphor technology-based lateral-flow assay[J]. Journal of Microbiological Methods, 2009, 79: 121-123.DOI:10.1016/j.mimet.2009.07.015.
[83] YONG Z, XIAO L, WANG X, et al. Development and evaluation of an up-converting phosphor technology-based lateral flow assay for rapid and quantitative detection of aflatoxin B1 in crops[J]. Talanta,2016, 161: 297-303. DOI:10.1016/j.talanta.2016.08.058.
[84] LIU X, ZHAO Y, SUN C Y, et al. Rapid detection of abrin in foods with an up-converting phosphor technology-based lateral flow assay[J].Scientific Reports, 2016, 6(1): 34926. DOI:10.1038/srep34926.
[85] 胡高爽, 张燕, 生威, 等. 上转换荧光猝灭试纸条检测牛奶中磺胺喹恶啉[J]. 食品科学, 2017, 38(24): 218-223. DOI:10.7506/spkx1002-6630-201724035.
[86] YAN Z Q, ZHOU L, ZHAO Y K, et al. Rapid quantitative detection of Yersinia pestis by lateral-flow immunoassay and up-converting phosphor technology-based biosensor[J]. Sensors & Actuators B Chemical, 2006, 119(2): 656-663. DOI:10.1016/j.snb.2006.01.029.
[87] SONG C, ZHI A, LIU Q, et al. Rapid and sensitive detection of β-agonists using a portable fluorescence biosensor based on fluorescent nanosilica and a lateral flow test strip[J]. Biosensors & Bioelectronics,2013, 50: 62-65. DOI: 10.1016/j.bios.2013.06.022.
[88] HUANG X, AGUILAR Z P, LI H, et al. Fluorescent Ru(phen)32+-doped silica nanoparticles-based ICTS sensor for quantitative detection of enrofloxacin residues in chicken meat[J]. Analytical Chemistry, 2013,85(10): 5120-5128. DOI:10.1021/ac400502v.
[89] WEI X, CHEN X, HUANG X, et al. Ru(phen)32+, doped silica nanoparticle based immunochromatographic strip for rapid quantitative detection of β-agonist residues in swine urine[J]. Talanta, 2013, 114:160-166. DOI:10.1016/j.talanta.2013.04.013.
[90] XIE Q Y, WU Y H, XIONG Q R, et al. Advantages of fluorescent microspheres compared with colloidal gold as a label in immunochromatographic lateral flow assays[J]. Biosensors &Bioelectronics, 2014, 54: 262-265. DOI:10.1016/j.bios.2013.11.002.
[91] LIU D, HUANG Y, CHEN M, et al. Rapid detection method for aflatoxin B1, in soybean sauce based on fluorescent microspheres probe[J]. Food Control, 2015, 50: 659-662. DOI:10.1016/j.foodcont.2014.10.011.
[92] WANG Z, LI H, LI C, et al. Development and application of a quantitative fluorescence-based immunochromatographic assay for fumonisin B1 in maize[J]. Journal of Agricultural and Food Chemistry,2014, 62(27): 6294-6298. DOI:10.1021/jf5017219.
[93] MAJDINASAB M, SHEIKH-ZEINODDIN M, SOLEIMANIAN-ZAD S,et al. A reliable and sensitive time-resolved fluorescent immunochromatographic assay (TRFICA) for ochratoxin A in agro-products[J]. Food Control, 2015, 47: 126-114. DOI:10.1016/j.foodcont.2014.06.044.
[94] WANG D B, TIAN B, ZHANG Z P, et al. Rapid detection of Bacillus anthracis spores using a super-paramagnetic lateral-flow immunological detection system[J]. Biosensors & Bioelectronics,2013, 42: 661-667. DOI:10.1016/j.bios.2012.10.088.
[95] WANG DB, TIAN B, ZHANG ZP, et al. Detection of Bacillus anthracis spores by super-paramagnetic lateral-flow immunoassays based on “Road Closure”[J]. Biosensors & Bioelectronics, 2015, 67:608-614. DOI:10.1016/j.bios.2014.09.067.
[96] 支援, 孟瑾, 郑小平, 等. 一种快速检测阪崎肠杆菌的新方法: 免疫磁性分离荧光标记[J]. 乳业科学与技术, 2010, 33(5): 231-233.DOI:10.15922/j.cnki.jdst.2010.05.010.
[97] SHI L, WU F, WEN Y, et al. A novel method to detect Listeria monocytogenes via superparamagnetic lateral flow immunoassay[J].Analytical & Bioanalytical Chemistry, 2015, 407(2): 529-535.DOI:10.1007/s00216-014-8276-8.
[98] RAEISOSSADATI M J, DANESH N M, BORNA F, et al. Lateral flow based immunobiosensors for detection of food contaminants[J].Biosensors & Bioelectronics, 2016, 86: 235-246. DOI:10.1016/j.bios.2016.06.061.
[99] LEE J Y, KIM Y A, KIM M Y, et al. Importance of membrane selection in the development of immunochromatographic assays for low-molecular weight compounds[J]. Analytica Chimica Acta, 2012,757(23): 69-74. DOI:10.1016/j.aca.2012.10.052.
[100] 谢艳君, 杨英, 孔维军, 等. 基于不同纳米材料的侧流免疫层析技术在真菌毒素检测中的应用[J]. 分析化学, 2015, 43(4): 618-628.DOI:10.11895/j.issn.0253-3820.141035.
Recent Progress on Nanoparticles in Lateral Flow Immunochromatographic Strip
TIAN Yachen, WANG Shujuan, MA Lan, XIE Manman, XU Dongpo, LIU Cheng, DING Chengchao, GUO Liang, FANG Shuiqin,WANG Xiang, QIU Jingxuan, DONG Qingli, LIU Qing*
(School of Medical Instrument and Food Engineering, University of Shanghai for Science and Technology, Shanghai 200093, China)
Abstract: Lateral flow immunoassay (LFIA), which combines the excellent separation ability of chromatography with the high specificity and sensitivity of conventional immunoassays, has served as an ideal platform for quantitative and sensitive on-site detection and played a significant role in food safety detection. The label is one of the key determinants of the sensitivity of LFIA. At present, the detection performance of LFIA is mainly enhanced by using nanoparticles (NPs) as labels including colored, luminescent and magnetic NPs. This paper is aimed to summarize the recent advances in the development of nanoparticle-based lateral flow immunochromatography. The application of various types of nanoparticles to improve the analytical performance is elaborated. This review is expected to provide powerful technical support for the selection of suitable nanoparticles for LFIA.Keywords: nanoparticles; label; lateral flow immunoassay
收稿日期:2018-08-19
基金项目:上海市科技创新行动计划项目(18495800400);上海市农委科技兴农项目( 沪农科推字(2017)第4-4号)
第一作者简介:田亚晨(1995—)(ORCID: 0000-0003-4287-5609),女,硕士研究生,研究方向为食品安全保障监控关键技术。E-mail: yayatyc@163.com
*通信作者简介:刘箐(1970—)(ORCID: 0000-0002-4988-9993),男,教授,博士,研究方向为食源性致病菌致病机理及快速检测技术。E-mail: liuq@usst.edu.cn
DOI:10.7506/spkx1002-6630-20180819-194
中图分类号:TS201.6
文献标志码:A
文章编号:1002-6630(2019)17-0348-09
引文格式:田亚晨, 王淑娟, 马兰, 等. 纳米颗粒在侧流免疫层析技术中的应用研究进展[J]. 食品科学, 2019, 40(17): 348-356.DOI:10.7506/spkx1002-6630-20180819-194. http://www.spkx.net.cn
TIAN Yachen, WANG Shujuan, MA Lan, et al. Recent progress on nanoparticles in lateral flow immunochromatographic strip[J]. Food Science, 2019, 40(17): 348-356. (in Chinese with English abstract) DOI:10.7506/spkx1002-6630-20180819-194.http://www.spkx.net.cn