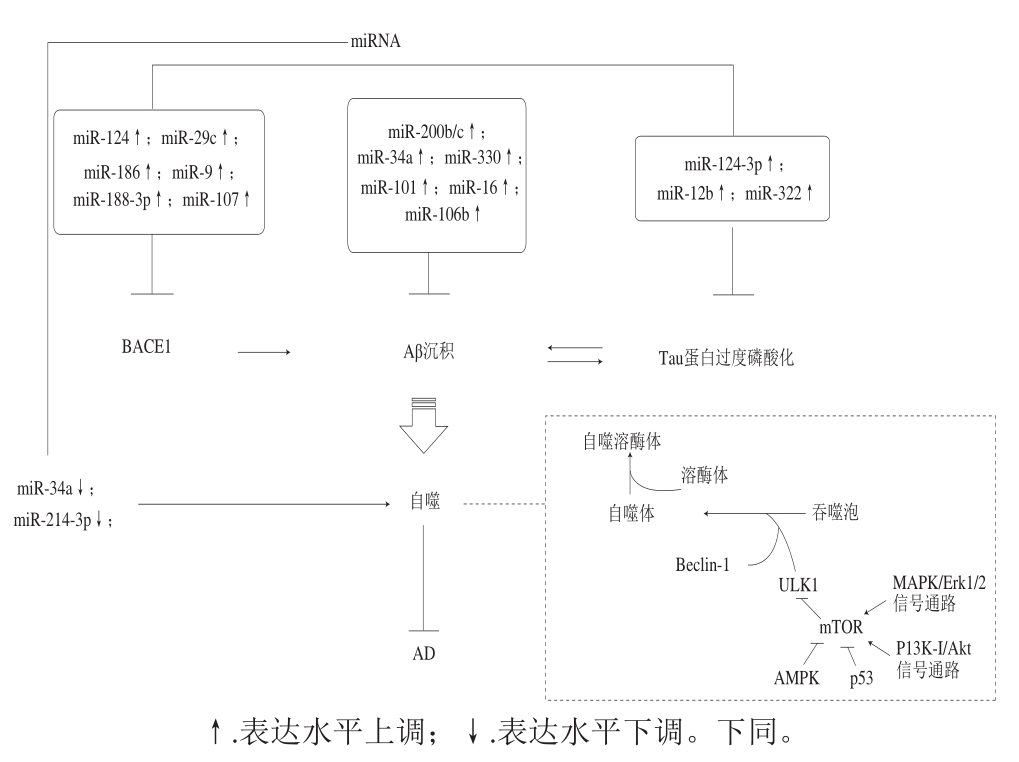
图 1 自噬与miRNA的相关信号通路
Fig. 1 Signaling pathways involved in the regulation of autophagy and miRNA
阿尔茨海默病(Alzheimer’s disease,AD)又称老年痴呆,是一种起病隐匿的进行性发展的神经系统退行性疾病,65 岁以前发病者称早老性痴呆,65 岁以后发病者称老年性痴呆,主要表现为渐进性记忆障碍、认知功能障碍、人格改变及语言功能障碍为主的神经精神症状,严重影响患者的生活、职业和社会交往。据国际AD协会发布的报告显示,2015—2050年,全球患有AD的人数将从4 600万 人增加至1.315亿 人[1],因此AD已经成为老年人常见的痴呆类型。AD的发病机制复杂又受遗传和环境因素的影响,并且随着AD的进展而导致相关生物分子水平的变化,目前关于AD的病因学说已有十几种,如β-淀粉样蛋白(β-amyloid protein,Aβ)沉积、Tau蛋白过度磷酸化、胆碱能缺陷、线粒体缺陷、神经细胞的凋亡、衰老与氧化应激、基因遗传、钙离子代谢紊乱以及雌激素缺陷等[2-4]。近年来,自噬与AD相关miRNA更加受到研究者的关注,研究发现其与神经传递功能障碍、突触功能障碍及认知缺陷都有密切关系,因而明确自噬和miRNA在AD中的调控与AD发病机制之间的联系对AD诊断与防治都有极大帮助。
众所周知,泛素-蛋白酶体系统(ubiquitinproteasome system,UPS)主要降解短寿命和可溶性蛋白质,而自噬溶酶体系统(autophagy-lysosome pathway,ALP)则可消化长寿命蛋白质复合物和损伤的细胞器。如果自噬功能异常,将会使异常或错误折叠的蛋白质聚集在细胞质、细胞核和细胞外包涵体中,导致神经元细胞器损伤和突触功能障碍,造成神经退行性疾病的发生[5-6],而随着年龄的增长,自噬活性表现出明显的减弱,自噬活性不足或减弱可导致有害蛋白质聚集体的形成和线粒体损伤的积累,从而相应地导致活性氧(reactive oxygen species,ROS)积累、细胞死亡和神经退行性疾病的增加[7-8]。自噬能够整合多种信号通路以调节细胞生长、细胞增殖、细胞运行和细胞存活,又接受雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)和腺苷酸活化蛋白激酶(AMP-activated protein kinase,AMPK)两大信号通路的严密调控。其中mTOR作为维持自噬功能的主要调节因子,存在两种不同功能的复合物:mTOR复合物1(mTOR compound 1,mTORC1)和mTOR复合物2(mTOR compound 2,mTORC2),mTORC1对雷帕霉素具有高度的敏感性,还可作为营养/能量/氧化还原的传感器,并控制蛋白质合成和运输;mTORC2通过促进蛋白激酶Cα的磷酸化调节细胞膜的骨架结构。除此之外激活AMPK通路可上调非依赖mTOR通路的自噬,在葡萄糖饥饿状态下,AMPK通过直接激活UNC-51样自噬激活激酶1(uncoordinated 51-like kinase 1,ULK1)促进自噬。因此,mTOR抑制剂和AMPK激活剂都可以用来提高自噬功能保护神经元损伤[9-11]。相反,由于自噬功能减弱造成的变性蛋白质的沉积及应激反应则会进一步阻碍自噬活性。另一方面从线粒体损伤学说来看,在营养缺乏、ROS蓄积、细胞衰老等外界刺激的作用下细胞内的线粒体会发生去极化并出现损伤,线粒体内的自噬缺陷可能会导致与神经变性有关的一系列神经元细胞的丢失,因此这种高度选择性自噬——线粒体自噬在神经元退行性改变中发挥着重要作用[12]。
miRNAs是一类内源性的非蛋白编码单链短序列RNA,转录后水平调控细胞内各类蛋白质的表达,并且miRNA在脑组织中含量十分丰富,能够调控神经系统中重要蛋白质的功能和各种生理过程,如:miR-124与miR-9a调控感觉神经元的发育和成熟,miR-134可以影响树突棘的形成,miR-132与miR-124在轴突生长过程中发挥不可或缺的作用,且miR-124的过表达可明显损害突触的活性[13-15]。因此miRNAs在中枢神经系统中异常表达(上调或下调)会影响其所调控靶基因的功能,造成中枢神经系统功能紊乱,最终导致神经退行性疾病的发生。幸运的是,研究者逐一找到了与AD样病理性蛋白密切相关的miRNA,发现在AD中均出现了此类miRNA的异常表达。AD的一个病理特征Aβ的形成主要由淀粉前体蛋白(amyloid precursor protein,APP)经β-分泌酶和γ-分泌酶剪切而产生的,研究发现AAP的表达量增多与miR-101、miR-330、miR-16、miR-200b/c表达减少或miR-128表达量增加有关[16-22],不仅APP表达量的增多可造成Aβ水平的增多,β-淀粉样前体蛋白裂解酶1(beta-site APP cleaving enzyme 1,BACE1)蛋白表达的增加也可以直接促进Aβ生成,而miR-15b、miR-195、miR-339-5p、miR-29c、miRN-124等[23-27]在神经细胞中的较低表达量则会导致BACE1蛋白表达显著上调,促使Aβ沉积加剧,Aβ本身的强神经毒性作用将造成突触功能失调、神经变性。另外miR-125b、miR-137、miR-124-3p、miR-132、miR-212、miR-322[28-32]可影响神经原纤维缠结(neurofibrillary tangles,NFT)的主要成分过磷酸化Tau蛋白表达水平,其中miR-124-3p、miR-34a可以直接提高自噬活性,本团队前期实验证实,在D-半乳糖(D-galactose,D-gal)诱导的大鼠脑衰老模型以及细胞衰老模型中,miR-34a通过调节细胞自噬相关蛋白(Atg7、Beclin1、LC3II/I、p62)以及线粒体动力学相关蛋白(Drp1和Mfn2)的表达参与调控大鼠脑衰老进程,可通过游泳运动激活miR-34a介导的细胞自噬改善线粒体功能,延缓脑衰老进程。除此之外,一些miRNA也可调控炎症和细胞凋亡过程。
当然miRNA和自噬也可通过调控细胞凋亡,氧化应激,基因水平等对AD的进程产生影响,但miRNA和自噬可直接调控AD病理特征蛋白表达水平,说明了miRNA和自噬是AD研究过程中不可忽视的靶点。其中,一方面自噬通过清除AD样病理性蛋白来延缓AD的进程,而自噬的启动可在变性蛋白堆积的情况下激活,但变性蛋白沉积过多超出了自噬可控的范围,则会造成自噬功能的障碍;另一方面miRNA通过调控AD病理样蛋白的表达提高自噬活性,其本身也可直接调控自噬相关蛋白的表达提高自噬活性,从而延缓AD(图1)。

图 1 自噬与miRNA的相关信号通路
Fig. 1 Signaling pathways involved in the regulation of autophagy and miRNA
随着人口老龄化的加剧,AD已经成为继心血管疾病,肿瘤和脑卒中之后的第四位杀手,目前抗痴呆药物的研究和开发已引起世界各国医药界的高度重视,近年来随着对老年神经生理、生化、药理等方面研究的不断深入,相关药物的开发研究不断取得进展,此类药物开发的新品数目超过了任何其他治疗类药物;然而,令人惋惜的是由于AD的发病机制非常复杂,到目前为止还没有特效药,并且绝大多数药物尚处于基础研究及临床实验阶段,因此医学研究者也开始关注治疗AD疾病的其他干预手段如运动、激素补给、天然产物等。目前已经有许多天然产物被证实对于AD的治疗有良好的效果,不仅在AD模型动物以及体外细胞实验中取得了极大的成功,而且部分已经投入临床使用在AD的治疗方面,越来越多的研究者发现这些天然产物还能够通过影响自噬进程和miRNA的表达,进而延缓神经退行性疾病(图2~3),提示天然产物通过调控自噬进程和miRNA的表达将成为治疗神经退行性疾病的突破口。
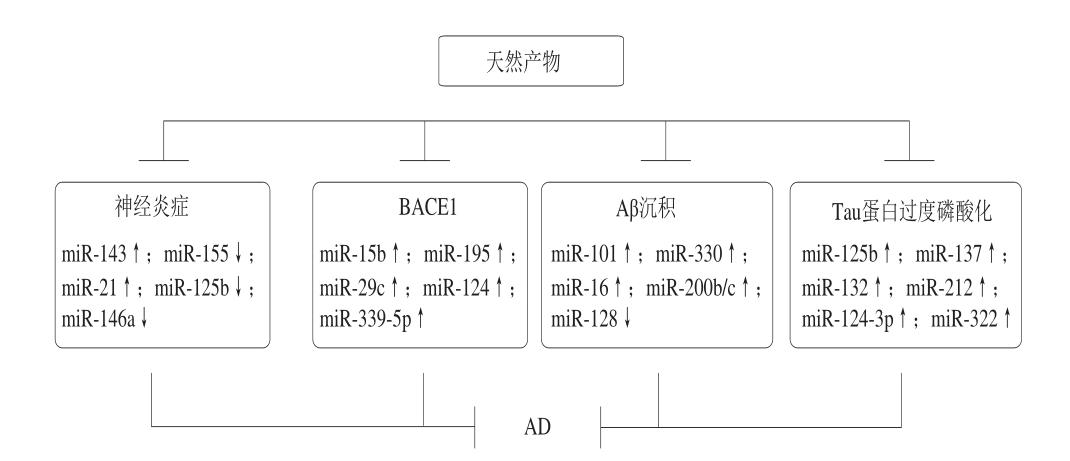
图 2 天然产物调控miRNA延缓AD
Fig. 2 Natural products regulate miRNA to delay AD
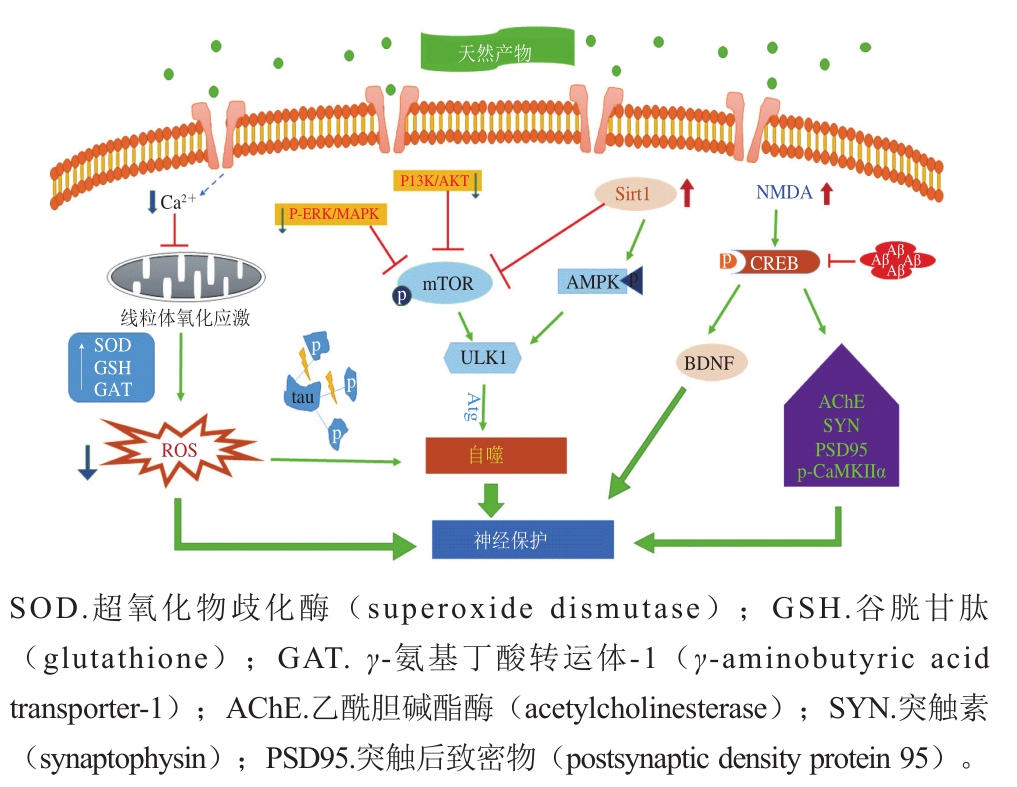
图 3 天然产物延缓AD相关信号通路
Fig. 3 Signaling pathway for natural products to delay the progression of AD
传统的治疗AD药物主要分为两类,一种是胆碱抑制剂,研究发现胆碱能神经元的减少与患者的认知功能障碍有密切关系,同时乙酰胆碱(acetylcholine,ACh)也参与短期记忆的形成,也是目前药物治疗的首选方式。另一种是N-甲基-D-天冬氨酸(N-methyl-D-aspartate,NMDA)受体抑制剂,此类药物可以拮抗NMDA受体,调节谷氨酸盐恢复到正常生理水平,此类药物研发较早,但仅对重度AD患者疗效显著,轻度患者则可能导致其他病症发生[34-37]。随着研究的不断进行也逐渐发现了其他辅助治疗药物,但由于这些药物仅针对单一症状疗效显著,而且带来的副作用较多,有些甚至患者难以承受,适用人群受限,导致这些药物无法被患者长期安全服用也难以推广。
2.2.1 二氢杨梅素
二氢杨梅素又名双氢杨梅素、蛇葡萄素,为二氢黄酮醇化合物,存在于葡萄科、杨梅科、杜鹃科、藤黄科、大戟科及柳科等植物中,其中在葡萄科藤茶含量最高。藤茶为葡萄科蛇葡萄属中的一种野生藤本植物,学名为显齿蛇葡萄(Ampelopsis grossedentata),又被俗称为客家白茶、龙藤茶、端午茶及土家神茶等,其因清热解毒、抗菌消炎、祛风除湿、强筋骨、降血压、降血脂、保肝护肝等功效深受人们的喜爱。目前研究者证实二氢杨梅素在清除自由基、抗氧化、抗血栓、抗肿瘤、抗衰老方面也具有良好的保护作用[38-39]。本团队的前期研究证明,蛇葡萄素能够激活PC12细胞(神经元替代模型)体内的血红素加氧酶1(hemeoxygenase-1,HO-1),使HO-1的表达量增加或酶活性增高从而起到保护PC12细胞免受过氧化氢诱导的神经细胞损伤,其机制在于蛇葡萄素能激活P13K-Akt和细胞外信号调节蛋白激酶1/2(extracellular regulated protein kinase 1/2,Erk1/2)信号通路[40],并且在6-OHDA诱导色神经细胞损伤的研究中,证实蛇葡萄素的神经保护作用是由于抑制了GSK-3β信号而使核转录相关因子2(nuclear factor(eryhroid-derived 2)-like 2 protein,Nrf2)表达增加,从而相应地抑制ROS和p-p38/p-JNK MAPK信号传导通路[41],而在内毒素诱导的巨噬细胞中也发现蛇葡萄素通过抑制ROS/Akt/IKK/核转录因子-κB信号通路减少NO和炎性因子的释放[42],鉴于前面一系列的研究均证实了蛇葡萄素有良好的抗炎和抗氧化特性,提示其在神经退行性疾病防治上有着很大的潜力。本团队最近的研究结果表明蛇葡萄素可以通过调控自噬和miRNA发挥神经保护作用,在D-gal诱导的大鼠脑衰老模型中,二氢杨梅素可通过上调AMPK/ULK1信号通路提高自噬功能,并促进沉默信息调节因子2相关酶1(sirtuin1,SIRT1)表达保护线粒体及其功能正常发挥的作用[43]。与此同时,D-gal诱导的脑衰老大鼠模型中miR-34a基因的异常高表达情况可以通过补充二氢杨梅素得以改善,其机制可能是通过抑制miR-34a表达上调SIRT1蛋白表达水平,进而抑制mTOR信号通路激活自噬,降低衰老相关蛋白p53和p21等表达水平从而延缓脑衰老[44]。
2.2.2 白藜芦醇
白藜芦醇是一种天然的非黄酮类多酚化合物,又称为芪三酚。主要来源于葡萄(红葡萄酒)、虎杖、花生、桑椹等植物,其中葡萄、葡萄皮中白藜芦醇的含量最高,可达50~100 mg/kg。白藜芦醇最先被人们熟知是作为一种天然的植物抗毒素,当葡萄等植株某个部位受到真菌感染、紫外线照射等不利条件作用时,该部位的白藜芦醇积累以应对不利条件,随后世界卫生组织调查发现,尽管法国人喜爱高脂食物,但冠心病发病率却较低,可能与其常饮含白藜芦醇的葡萄酒有关。因此白藜芦醇的抗心血管疾病、抗肿瘤和抗氧化作用被广泛认可,目前研究者们证实白藜芦醇在神经退行性疾病中发挥保护作用[45-46]。研究表明白藜芦醇作为长寿蛋白SIRT1激活剂,促进SIRT1蛋白的表达水平,其中SIRT1作为脱乙酰基酶,能使能使肝激酶B1蛋白(liver kinase B1,LKB1)去乙酰化来促进AMPK的磷酸化,促进自噬激活,而且SIRT1还可使自噬重要蛋白Atg5、Atg7和LC3去乙酰化,促进自噬进程,从而形成SIRT1/LKB1/AMPK信号通路激活自噬[47-49]。除此之外,白藜芦醇还可以抑制由应激反应损伤激活的PI3K/Akt/mTOR途径来激活自噬,因此,有研究者提出mTOR可作为白藜芦醇激活自噬的一个新的靶点[50-51],并且在AD模型SD大鼠中发现自噬抑制剂氯喹不仅可以消除白藜芦醇的预防作用,还能使白藜芦醇转化为有害物质产生炎症反应[52-53],因此,白藜芦醇通过激活自噬延缓AD是得到广泛认可的。然而白藜芦醇在调控miRNA延缓AD方面还在起步阶段,但研究显示白藜芦醇可以调控与AD密切相关的miRNA发挥保护神经系统的作用。在自然衰老小鼠中,人们发现白藜芦醇提高miR-134/124的表达激活miRNA/Sirt1通路,进而上调其在海马下游信号环磷腺苷效应元件结合蛋白(cAMP-response element binding protein,CREB)表达水平,从而促进脑源性神经营养因子(brain derived neurotropic factor,BDNF)的合成,形成miRNA134/124-CREB-BDNF信号通路延缓中枢神经衰老及认知功能减弱[54-55]。在神经毒素1-甲基-4-苯基-1,2,3,6-四氢吡啶诱导的帕金森病小鼠及1-甲基-4-苯基吡啶处理的SH-SY5Y细胞中发现miR-214表达水平较低,α-突触核蛋白mRNA和蛋白表达水平较高,白藜芦醇处理后可以上调miR-214抑制α-突触核蛋白表达[56]。除此之外,在神经退行性疾病中发现miR-21、miR-155、miR-125b和miR-146a显著性上调,可能与加重神经炎症密切相关,白藜芦醇通过上调miR-34a抑制核转录因子STAT3磷酸化,阻碍缺氧诱导的胶质瘤细胞迁移和侵袭,也通过上调miR-126抑制PI3K/Akt激活mTOR途径,保护内皮细胞免受氧化损伤[57-59]。其次,白藜芦醇能够通过增强miR-106b的表达、下调AD病理样蛋白(APP、Tau)的表达改善认知功能[60],但此观点还需进一步研究证实。
2.2.3 姜黄素
姜黄素为二酮类化合物,主要从姜科、天南星科中的一些植物的根茎中提取,饮食中生姜、芥末、咖哩等也富含姜黄素,并且姜黄素长期以来就作为一种常用的天然色素被广泛地应用在食品工业中,主要用于罐头、肠类制品、酱卤制品的染色,是世界卫生组织和美国食品药品管理局以及多国准许使用的食品添加剂。早期的研究表明其具有抗炎、抗氧化、调脂、抗病毒、抗感染、抗肿瘤、抗凝、抗肝纤维化、抗动脉粥样硬化等广泛的药理活性,且毒副作用很小[61]。近来研究发现姜黄素可增强脑组织神经突触活性,通过磷酸化钙/钙调素依赖蛋白激酶II α(calcium/calmodulin-dependent protein kinaseII α,CaMKII α)、突触后致密物(PSD-95)和突触素等表达水平,同时提高BDNF、p-GSK-3β蛋白表达,形成PI3K/Akt/GSK-3β信号通路减少亚砷酸钠诱导的海马区改变[62],也可通过抑制超氧化物歧化酶和谷胱甘肽过氧化物酶活性及抑制乙酰胆碱酯酶活化抑制铝诱导的大鼠脑组织氧化损伤和凋亡[63]。但只在SH-SY5Y细胞中发现姜黄素提高NMDA受体活性,阻止NMDA受体介导的细胞内Ca2+水平升高,抑制Aβ诱导的人神经母细胞瘤SH-SY5Y细胞损伤和死亡[64]。姜黄素对于神经系统的保护作用已经被多数体外和体内研究认可,其通过自噬发挥神经保护作用是由多种信号途径介导,包括PI3K/Akt/mTOR、AMPK/ULK1、MAPK/ERK1/2等[65-68]信号通路以及上调miR-143激活自噬,减少ROS积累,抑制氧化损伤或上调miR-145抑制PI3K/Akt/mTOR途径[69-70]。因此研究姜黄素诱导的自噬作用是否能够以预定的剂量和持续时间选择性地靶向损伤细胞具有重要意义。
2.2.4 其他
至今为止,从食品中提取出能够延缓AD进程的天然产物尚少,其他潜在的天然产物也尚在研究过程中,如:人参皂苷在我国主要以野生参和园参为主,野生参稀少珍贵,但随着种植技术的大幅改进,园参的产量逐年提高,根据加工方式不同主要以生晒参、白参、红参见于市场;而饮食中常见的牛蒡也是参类的一种,早年移植到日本凭借其独特的香气和纯正的口味,走俏东南亚,俗称为“东洋参”。其次,由欧洲引进的西洋参也常用来泡水喝和作为煲汤的辅料。人参皂苷具有的消除自由基,抗氧化,抗心血管疾病以及延缓细胞衰老,保护神经系统,提高老年人记忆力等功能均获得一致的认可。人参皂苷延缓神经退行性疾病主要作用在提高突触相关蛋白表达水平,增强突触可塑性[71];上调CREB与BDNF表达水平,减少细胞凋亡[72-73]与AD病理样蛋白表达,清除自由基,保护神经细胞[74-76];而目前发现人参皂苷可通过上调PI3K/Akt信号通路,减少Aβ处理的PC12细胞凋亡,可能是由于人参皂苷降低了活性氧和细胞内Ca2+浓度,激活自噬通路[77-78]。研究发现人参皂苷还可通过上调miR-134改善慢性应激大鼠的抑郁行为,人参皂苷促进小鼠脂肪源性干细胞的神经分化可能通过miR-124信号通路,但其具体机制尚不清楚[80]。大蒜素又叫大蒜新素,是从蒜的球形鳞茎中提取的挥发性油状物,为二烯丙基三硫化物、二烯丙基二硫化物以及甲基烯丙基二硫化物等的混合物,具有较好的脂溶性,可通过血脑屏障、清除自由基、抗氧化延缓衰老的作用,其保护海马神经元改善AD的作用在于抑制氧化损伤减少Tau蛋白磷酸化[81],Dong Juan等[82]提出大蒜素可降低ERK1/2磷酸化及其下游底物核转录因子Nrf2的表达,抑制氧化应激反应,并且在H2O2诱导PC12细中也证实了大蒜素抗氧化保护神经细胞的作用[83]。除此之外,许多从中药植物中提取的天然产物均被证明具有良好的抗神经退行性疾病的作用,如:蛇床子素、黄芩素、原花青素、银杏提取物、查耳酮类衍生物等,但还需更多的研究证实天然产物在保护神经系统中发挥的重要作用。
虽然AD目前为世界难题,但许多治疗手段也逐渐得到一定认可,而天然产物通过调控自噬和miRNA发挥治疗AD的作用已经得出了一定的明确结论,相信在临床上的应用将指日可待,并且随着研究发现miRNA在AD患者脑脊液内的异常表达具有部位特异性和阶段特异性,表明miRNA有望成为方便有效的生物标志物来帮助临床诊断[84-85],以及作为天然产物治疗神经退行性疾病的可靠依据。虽然AD的病因十分复杂且各种病因相互影响,但是研究者也在不断地探索更多有效的方法,最近提出的“突触丢失=AD”的新观点[86-87]也将成为AD研究的新思路,因此通过政府与研究人员的不断努力,新颖而有效的AD干预办法也将逐渐被开发并服务于大众。
[1] Alzheimer’s Disease International. World Alzheimer’s report 2015: global impact of dementia[M]. London: Alzheimer’s Disease International, 2015: 1-79.
[2] KLEMENTIEVA O, WILLÉN K, MARTINSSON I, et al.Pre-plaque conformational changes in Alzheimer’s diseaselinked Aβ and APP[J]. Nature Communications, 2017, 8: 1-16.DOI:10.1038/ncomms14726.
[3] ZOLTOWSKA K M, BEREZOVSKA O. Dynamic nature of presenilin1/γ-secretase: implication for Alzheimer’s disease pathogenesis[J]. Molecular Neurobiology, 2018, 55(6): 1-10.DOI:10.1007/s12035-017-0487-5.
[4] GOETZL E J, SCHWARTZ J B, ABNER E L, et al. High complement levels in astrocyte-derived exosomes of Alzheimer’s disease[J]. Annals of Neurology, 2018, 83(3): 544-552. DOI:10.1002/ana.25172.
[5] NAKAMURA A, KANEKO N, VILLEMAGNE V L, et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease[J].Nature, 2018, 554: 249-254. DOI:10.1038/nature25456.
[6] ZHANG Y, CHEN X, ZHAO Y F, et al. The role of ubiquitin proteasomal system and autophagy-lysosome pathway in Alzheimer’s disease[J]. Reviews in the Neurosciences, 2017, 28(8): 861-868.DOI:10.1515/revneuro-2017-0013.
[7] LI Q, LIU Y, SUN M. Autophagy and Alzheimer’s disease[J].Cellular & Molecular Neurobiology, 2016, 37(3): 1-12. DOI:10.1007/s10571-016-0386-8.
[8] NIXON R A. The role of autophagy in neurodegenerative disease[J].Nature Medicine, 2013, 19(8): 983-997. DOI:10.1038/nm.3232.
[9] KIM J, KUNDU M, VIOLLET B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1[J]. Nature Cell Biology, 2011, 13(2): 132-141. DOI:10.1038/ncb2152.
[10] SHANG L B, WANG X D. AMPK and mTOR coordinate the regulation of Ulk1 and mammalian autophagy initiation[J]. Autophagy,2011, 7(8): 924-926.
[11] FAN K R, LIN L, AI Q, et al. Lipopolysaccharide-induced dephosphorylation of AMPK-activated protein kinase potentiates inflammatory injury via repression of ULK1-dependent autophagy[J]. Frontiers in Immunology, 2018, 9: 1-9. DOI:10.3389/fimmu.2018.01464.
[12] AMADORO G, CORSETTI V, ATLANTE A, et al. An Alzheimer’slinked toxic NH2-fragment of human tau affects the parkin-driven mitophagy in primary hippocampal neurons[J]. Alzheimers &Dementia, 2014, 10(4): 647-647.
[13] STAPPERT L, ROESE-KOERNER B, BRÜSTLE O. The role of microRNAs in human neural stem cells, neuronal differentiation and subtype specification[J]. Cell & Tissue Research, 2015, 359(1): 47-64.DOI:10.1007/s00441-014-1981-y.
[14] LAMBERT T J, STORM D R, SULLIVAN J M. MicroRNA-132 modulates short-term synaptic plasticity but not basal release probability in hippocampal neurons[J]. PLoS ONE, 2010, 5(12):e15182. DOI:10.1371/journal.pone.0015182.
[15] WANG X, LIU D, HUANG H Z, et al. A novel microRNA-124/PTPN1 signal pathway mediates synaptic and memory deficits in Alzheimer’s disease[J]. Biological Psychiatry, 2018, 83(5): 395-405.DOI:10.1016/j.biopsych.2017.07.023.
[16] VILARDO E, BARBATO C, CIOTTI M, et al. MicroRNA-101 regulates amyloid precursor protein expression in hippocampal neurons[J]. Journal of Biological Chemistry, 2010, 285(24): 18344-18351. DOI:10.1074/jbc.M110.112664.
[17] LONG J M, LAHIRI D K. MicroRNA-101 downregulates Alzheimer’s amyloid-β precursor protein levels in human cell cultures and is differentially expressed[J]. Biochemical and Biophysical Research Communications, 2011, 404(4): 889-895. DOI:10.1016/j.bbrc.2010.12.053.
[18] ZHOU Y, WANG Z F, LI W, et al. Protective effects of microRNA-330 on amyloid β-protein production, oxidative stress, and mitochondrial dysfunction in Alzheimer’s disease by targeting VAV1 via the MAPK signaling pathway[J]. Journal of Cellular Biochemistry,2018, 119(7): 5437-5448. DOI:10.1002/jcb.26700.
[19] LIU W, LIU C, ZHU J X, et al. MicroRNA-16 targets amyloid precursor protein to potentially modulate Alzheimer’s-associated pathogenesis in SAMP8 mice[J]. Neurobiology of Aging, 2012, 33(3):522-534. DOI:10.1016/j.neurobiolaging.2010.04.034.
[20] HIGAKI S, MURAMATSU M, MATSUDA A, et al. Defensive effect of microRNA-200b/c against amyloid-beta peptide-induced toxicity in Alzheimer’s disease models[J]. PLoS ONE, 2018, 13(5): e0196929.
[21] TIRIBUZI R, CRISPOLTONI L, PORCELLATI S, et al. miR-128 up-regulation correlates with impaired amyloid β(1-42) degradation in monocytes from patients with sporadic Alzheimer’s disease.[J].Neurobiology of Aging, 2013, 9(4): 195-195. DOI:10.1016/j.neurobiolaging.
[22] GENG L J, ZHANG T, LIU W, et al. Inhibition of miR-128 abates Aβmediated cytotoxicity by targeting PPAR-γ via NF-κB inactivation in primary mouse cortical neurons and neuro2a cells[J]. Yonsei Medical Journal, 2018, 59(9): 1096-1106. DOI:10.3349/ymj.2018.59.9.1096.
[23] LI J, WANG H T. miR-15b reduces amyloid-β accumulation in SH-SY5Y cell line through targeting NF-κB signaling and BACE1[J].Bioscience Reports, 2018, 38(6): 1-10. DOI:10.1042/BSR20180051.
[24] ZHU H C, WANG L M, WANG M, et al. MicroRNA-195 downregulates Alzheimer’s disease amyloid-β production by targeting BACE1[J]. Brain Research Bulletin, 2012, 88(6): 596-601.DOI:10.1016/j.brainresbull.
[25] LONG J M, RAY B, LAHIRI D K. MicroRNA-339-5p down-regulates protein expression of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) in human primary brain cultures and is reduced in brain tissue specimens of Alzheimer disease subjects[J]. Journal of Biological Chemistry, 2014, 289(8): 5184-5198. DOI:10.1074/jbc.M113.518241.
[26] LEI X F, LEI L J, ZHANG Z L, et al. Downregulated miR-29c correlates with increased BACE1 expression in sporadic Alzheimer’s disease[J]. International Journal of Clinical and Experimental Pathology, 2014, 8(2): 1565-1574.
[27] FANG M R, WANG J, ZHANG X B, et al. The miR-124 regulates the expression of BACE1/β-secretase correlated with cell death in Alzheimer’s disease[J]. Toxicology Letters, 2012, 209(1): 94-105.DOI:10.1016/j.toxlet.2011.11.032.
[28] BANZHAF-STRATHMANN J, BENITO E, MAY S, et al.MicroRNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer’s disease[J]. EMBO Journal, 2014, 33(15):1667-1680. DOI:10.15252/embj.201387576.
[29] JIANG Y, XU B, CHEN J, et al. Micro-RNA-137 inhibits tau hyperphosphorylation in Alzheimer’s disease and targets the CACNA1C gene in transgenic mice and human neuroblastoma SH-SY5Y cells[J]. Medical Science Monitor, 2018, 24: 5635-5644.DOI:10.12659/MSM.908765.
[30] KANG Q M, XIANG Y, LI D, et al. MiR-124-3p attenuates hyperphosphorylation of tau protein-induced apoptosis via caveolin-1-PI3K/Akt/GSK3β pathway in N2a/APP695swe cells[J]. Oncotarget,2017, 8(15): 24314-24326. DOI:10.18632/oncotarget.15149.
[31] EL FATIMY R, LI S, CHEN Z, et al. MicroRNA-132 provides neuroprotection for tauopathies via multiple signaling pathways[J].Acta Neuropathologica, 2018, 136(4): 537-555. DOI:10.1007/s00401-018-1880-5.
[32] WANG Y, VEREMEYKO T, WONG A H, et al. Downregulation of miR-132/212 impairs S-nitrosylation balance and induces tau phosphorylation in Alzheimer’s disease[J]. Neurobiol Aging, 2017, 51:156-166. DOI:10.1016/j.neurobiolaging.
[33] ZHANG J, LIU Z C, PEI Y Y, et al. MicroRNA-322 cluster promotes tau phosphorylation via targeting brain-derived neurotrophic factor[J].Neurochemical Research, 2018, 43(3): 736-744. DOI:10.1007/s11064-018-2475-1.
[34] KOU X J, LI J, LIU X R, et al. Swimming attenuates D-galactoseinduced brain aging via suppressing miR-34a-mediated autophagy impairment and abnormal mitochondrial dynamics[J]. Journal of Applied Physiology, 2017, 122(6): 1462-1469. DOI:10.1152/japplphysiol.00018.
[35] SOTO M, ANDRIEU S, NOURHASHEMI F, et al. Medication development for agitation and aggression in Alzheimer disease:review and discussion of recent randomized clinical trial design[J].International Psychogeriatrics, 2015, 27(2): 181-197. DOI:10.1017/S1041610214001720.
[36] BEIER M T. Treatment strategies for the behavioral symptoms of Alzheimer’s disease: focus on early pharmacologic intervention[J].Pharmacotherapy, 2012, 27(3): 399-411. DOI:10.1592/phco.27.3.399.
[37] KNAPP M, KING D, ROMEO R, et al. Cost-effectiveness of donepezil and memantine in moderate to severe Alzheimer’s disease(the DOMINO-AD trial)[J]. International Journal of Geriatric Psychiatry, 2017, 32(12): 1205-1216. DOI:10.1002/gps.4583.
[38] ARAKI T, WAKE R, MIYAOKA T, et al. The effects of combine treatment of memantine and donepezil on Alzheimer’s disease patients and its relationship with cerebral blood fl ow in the prefrontal area[J].International Journal of Geriatric Psychiatry, 2014, 29(9): 881-889.DOI:10.1002/gps.4074.
[39] 徐静娟, 姚茂君, 许钢. 二氢杨梅素抗氧化功能的研究[J]. 食品科学,2007, 28(9): 43-45.
[40] 侯小龙, 王文清, 施春阳, 等. 二氢杨梅素药理作用研究进展[J]. 中草药, 2015, 46(4): 603-609.
[41] KOU X J, SHEN K Y, AN Y H, et al. Ampelopsin inhibits H2O2-induced apoptosis by ERK and Akt signaling pathways and upregulation of heme oxygenase-1[J]. Phytotherapy Research, 2012,26(7): 988-994. DOI:10.1002/ptr.3671.
[42] KOU X J, LI J, BIAN J, et al. Ampelopsin attenuates 6-OHDA-induced neurotoxicity by regulating GSK-3β/NRF2/ARE signalling[J]. Journal of Functional Foods, 2015, 19: 765-774.
[43] QI S M, XIN Y Q, GUO Y T, et al. Ampelopsin reduces endotoxic inflammation via repressing ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways[J]. International Immunopharmacology,2012, 12(1): 278-287. DOI:10.1016/j.intimp.2011.12.001.
[44] KOU X J, LI J, LIU X R, et al. Ampelopsin attenuates the atrophy of skeletal muscle from D-gal-induced aging rats through activating AMPK/SIRT1/PGC-1α signaling cascade[J].Biomedicine & Pharmacotherapy, 2017, 90: 311-320. DOI:10.1016/j.biopha.2017.03.070.
[45] KOU X J, LIU X R, CHEN X B, et al. Ampelopsin attenuates brain aging of D-gal-induced rats through miR-34a-mediatedSIRT1/mTOR signal pathway[J]. Oncotarget, 2016, 7(46): 74484-74495.DOI:10.18632/oncotarget.12811.
[46] 张泽生, 贺伟, 刘甜甜, 等. 白藜芦醇的体外抗氧化活性[J]. 食品科学, 2012, 33(11): 266-268.
[47] 刘贵珊, 杨博, 张泽生, 等. 白藜芦醇对D-半乳糖致衰老小鼠学习记忆能力和脑组织抗氧化能力的影响[J]. 食品科学, 2014, 35(5): 204-207. DOI:10.7506/spkx1002-6630-201405040.
[48] 陈思凡, 肖新才, 孙延双, 等. 白藜芦醇对大鼠脂肪细胞抗氧化及胰岛素敏感性的影响[J]. 食品科学, 2010, 31(13): 263-266.
[49] KOU X J, CHEN N. Resveratrol as a natural autophagy regulator for prevention and treatment of Alzheimer’s disease[J]. Nutrients, 2017, 9:1-13. DOI:10.3390/nu9090927.
[50] CAO W Y, DOU Y, LI A P. Resveratrol boosts cognitive function by targeting SIRT1[J]. Neurochemical Research, 2018, 43(9): 1705-1713.DOI:10.1007/s11064-018-2586-8.
[51] JIA Y M, WANG N, LIU X W. Resveratrol and amyloid-beta:mechanistic insights[J]. Nutrients, 2017, 9(10): 1-13. DOI:10.3390/nu9101122.
[52] GAO J L, ZHANG Q Y, SONG L. Matrix biosynthesis of nucleus pulposus cells through activating autophagy via the PI3K/Akt pathway under oxidative damage[J]. Bioscience Reports, 2018, 38(4):BSR20180544. DOI:10.1042/BSR20180544.
[53] PARK D, JEONG H, LEE M N, et al. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition[J]. Scientific Reports, 2016, 6: 1-11. DOI:10.1038/srep21772.
[54] ULAKCSAI Z, BAGAMÉRY F, SZÖKŐ É, et al. The role of autophagy induction in the mechanism of cytoprotective effect of resveratrol[J]. European Journal of Pharmaceutical Sciences, 2018,123: 135-142. DOI:10.1016/j.ejps.2018.07.039.
[55] MENG H Y, SHAO D C, LI H, et al. Resveratrol improves neurological outcome and neuroinflammation following spinal cord injury through enhancing autophagy involving the AMPK/mTOR pathway[J]. Molecular Medicine Reports, 2018, 18(2): 2237-2244.DOI:10.3892/mmr.2018.9194.
[56] SHEN J, XU L L, QU C J, et al. Resveratrol prevents cognitive deficits induced by chronic unpredictable mild stress: Sirt1/miR-134 signalling pathway regulates CREB/BDNF expression in hippocampus in vivo and in vitro[J]. Behavioural Brain Research, 2018, 349(3): 1-7.DOI:10.1016/j.bbr.2018.04.050.
[57] ZHAO Y N, LI WF, LI F, et al. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway[J].Biochemical and Biophysical Research Communications, 2013,435(4): 597-602. DOI:10.1016/j.bbrc.
[58] WANG Z H, ZHANG J L, DUAN Y L, et al. MicroRNA-214 participates in the neuroprotective effect of resveratrol via inhibiting α-synuclein expression in MPTP-induced Parkinson’s disease mouse[J]. Biomedicine & Pharmacotherapy, 2015, 74: 252-256.DOI:10.1016/j.biopha.2015.08.025.
[59] WANG H, FENG H, ZHANG Y. Resveratrol inhibits hypoxia-induced glioma cell migration and invasion by the p-STAT3/miR-34a axis[J].Neoplasma, 2016, 63(4): 532-539. DOI:10.4149/neo_2016_406.
[60] ZENG K, WANG Y, YANG N, et al. Resveratrol inhibits diabeticinduced Müller cells apoptosis through microRNA-29b/specificity protein 1 pathway[J]. Molecular Neurobiology, 2017, 54(6): 4000-4014. DOI:10.1007/s12035-016-9972-5.
[61] SUI X Q, XU Z M, XIE M B, et al. Resveratrol inhibits hydrogen peroxide-induced apoptosis in endothelial cells via the activation of PI3K/Akt by miR-126[J]. Journal of Atherosclerosis and Thrombosis,2014, 21(2): 108-118.
[62] BRAIDY N, JUGDER B E, POLJAK A, et al. Resveratrol as a potential therapeutic candidate for the treatment and management of Alzheimer’s disease[J]. Current Topics in Medicinal Chemistry, 2016,16(17): 1951-1960.
[63] 薛海鹏, 李湘洲, 旷春桃, 等. 姜黄素的抗氧化机制及以其为先导物的抗氧化化合物研究进展[J]. 食品科学, 2010, 31(7): 302-307.
[64] SRIVASTAVA P, DHURIYA YK, KUMAR V, et al. PI3K/Akt/GSK3β induced CREB activation ameliorates arsenic mediated alterations in NMDA receptors and associated signaling in rat hippocampus:neuroprotective role of curcumin[J]. Neurotoxicology, 2018, 67: 190-205. DOI:10.1016/j.neuro.2018.04.018.
[65] TÜZMEN M N, YÜCEL N C, KALBURCU T, et al. Effects of curcumin and tannic acid on the aluminum- and lead-induced oxidative neurotoxicity and alterations in NMDA receptors[J]. Toxicology Methods, 2015, 25(2): 120-127. DOI:10.3109/15376516.2014.997947.
[66] MAITI P, DUNBAR G L. Comparative neuroprotective effects of dietary curcumin and solid lipid curcumin particles in cultured mouse neuroblastoma cells after exposure to Aβ42[J]. International Journal of Alzheimer’s Disease, 2017, 2017: 1-13. DOI:10.1155/2017/4164872.
[67] SEO S U, WOO S M, LEE H S, et al. mTORC1/2 inhibitor and curcumin induce apoptosis through lysosomal membrane permeabilization-mediated autophagy[J]. Oncogene, 2018, 37(38):5205-5220. DOI:10.1038/s41388-018-0345-6.
[68] SONG G Q, LU H H, CHEN F, et al. Tetrahydrocurcumin-induced autophagy via suppression of PI3K/Akt/mTOR in non-small cell lung carcinoma cells[J]. Molecular Medicine Reports, 2018, 17(4): 5964-5969. DOI:10.3892/mmr.2018.8600.
[69] LI X D, FENG K, LI J, et al. Curcumin inhibits apoptosis of chondrocytes through activation ERK1/2 signaling pathways induced autophagy[J]. Nutrients, 2017, 9: 1-14. DOI:10.3390/nu9040414.
[70] WANG J L, WANG J J, CAI Z N, et al. The effect of curcumin on the differentiation, apoptosis and cell cycle of neural stem cells is mediated through inhibiting autophagy by the modulation of Atg7 and p62[J]. International Journal of Molecular Medicine, 2018, 42(5):2481-2488. DOI:10.3892/ijmm.2018.3847.
[71] LIU J B, LI M, WANG Y W, et al. Curcumin sensitizes prostate cancer cells to radiation partly via epigenetic activation of miR-143 and miR-143 mediated autophagy inhibition[J]. Journal of Drug Targeting,2017, 25(7): 645-652. DOI:10.1080/1061186X.2017.1315686.
[72] ZHU X F, ZHU R H. Curcumin suppresses the progression of laryngeal squamous cell carcinoma through the upregulation of miR-145 and inhibition of the PI3K/Akt/mTOR pathway[J]. OncoTargets and Therapy, 2018, 11: 3521-3531. DOI:10.2147/OTT.S159236.
[73] NIE L L, XIA J X, LI H L, et al. Ginsenoside Rg1 ameliorates behavioral abnormalities and modulates the hippocampal proteomic change in triple transgenic mice of Alzheimer’s disease[J].Oxidative Medicine and Cellular Longevity, 2017, 2017: 1-17.DOI:10.1155/2017/6473506.
[74] YOU Z C, YAO Q, SHEN J H, et al. Antidepressant-like effects of ginsenoside Rg3 in mice via activation of the hippocampal BDNF signaling cascade[J]. Journal of Natural Medicines, 2017, 71(2):367-379. DOI:10.1007/s11418-016-1066-1.
[75] ZHU X Z, GAO R, LIU Z X, et al. Ginsenoside Rg1 reverses stressinduced depression-like behaviours and brain-derived neurotrophic factor expression within the prefrontal cortex[J]. European Journal of Neuroscience, 2016, 44(2): 1878-1885. DOI:10.1111/ejn.13255.
[76] FUJIHARA K, KOIKE S, OGASAWARA Y, et al. Inhibition of amyloid β aggregation and protective effect on SH-SY5Y cells by triterpenoid saponins from the cactus Polaskia chichipe[J]. Bioorganic &Medicinal Chemistry, 2017, 25(13): 3377-3383. DOI:10.1016/j.bmc.2017.04.023.
[77] CAO G Q, SU P, ZHANG S, et al. Ginsenoside rereduces Aβ production by activating PPARγ to inhibit BACE1 in N2a/APP695 cells[J]. European Journal of Pharmacology, 2016, 793: 101-108.DOI:10.1016/j.ejphar.2016.11.006.
[78] GONZÁLEZ-BURGOS E, FERNÁNDEZ-MORIANO C, LOZANO R,et al. Ginsenosides Rd and Re co-treatments improve rotenoneinduced oxidative stress and mitochondrial impairment in SH-SY5Y neuroblastoma cells[J]. Food and Chemical Toxicology, 2017, 109(1):38-47. DOI:10.1016/j.fct.2017.08.013.
[79] CUI J, WANG J, ZHENG M Z, et al. Ginsenoside Rg2 protects PC12 cells against β-amyloid25-35-induced apoptosis via the phosphoinositide 3-kinase/Akt pathway[J]. Chemico-Biological Interactions, 2017, 275:152-161. DOI:10.1016/j.cbi.2017.07.021.
[80] TU T T, SHARMA N, SHIN E J, et al. Ginsenoside Re protects trimethyltin-induced neurotoxicity via activation of IL-6-mediated phosphoinositol 3-kinase/Akt signaling in mice[J]. Neurochemical Research, 2017, 42(11): 1-15. DOI:10.1007/s11064-017-2349-y.
[81] FAN C Q, ZHU X Z, SONG Q Q, et al. MiR-134 modulates chronic stress-induced structural plasticity and depressionlike behaviors via downregulation of Limk1/cofilin signaling in rats[J]. Neuropharmacology, 2018, 131: 364-376. DOI:10.1016/j.neuropharm.2018.01.009.
[82] DONG Juan, ZHU Guo, WANG Tiancheng, et al. Ginsenoside Rg1 promotes neural differentiation of mouse adipose-derived stem cells via the miRNA-124 signaling pathway[J]. Journal of Zhejiang University-SCIENCE B, 2017, 18(5): 445-448. DOI:10.1631/jzus.B1600355.
[83] 张浩, 潘海, 王玥. 大蒜素对阿尔茨海默病转基因小鼠脑内Tau蛋白表达的影响[J]. 天然产物研究与开发, 2016(5): 685-689.
[84] ZHU Y F, LI X H, YUAN Z P, et al. Allicin improves endoplasmic reticulum stress-related cognitive deficits via PERK/Nrf2 antioxidative signaling pathway[J]. European Journal of Pharmacology, 2015,762(1): 239-246. DOI:10.1016/j.ejphar.2015.06.002.
[85] LÜ R X, DU L L, LU C W, et al. Allicin protects against H2O2-induced apoptosis of PC12 cells via the mitochondrial pathway[J].Experimental and Therapeutic Medicine, 2017, 14(3): 2053-2059.DOI:10.3892/etm.2017.4725.
[86] ELLIOTT C, ROJO A I, RIBE E, et al. A role for APP in Wnt signalling links synapse loss with β-amyloid production[J]. Translational Psychiatry,2018, 8(1): 1-13. DOI:10.1038/s41398-018-0231-6.
[87] KAMAT P K, KALANI A, RAI S, et al. Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease:understanding the therapeutics strategies[J]. Molecular Neurobiology,2016, 53(1): 648-661. DOI:10.1007/s12035-014- 9053-6.
Natural Products Alleviate Alzheimer’s Disease through Regulating miRNA and the Autophagy Signal Pathway
CHEN Dandan, CHEN Ning, KOU Xianjuan. Natural products alleviate Alzheimer’s disease through regulating miRNA and the autophagy signal pathway[J]. Food Science, 2019, 40(5): 295-302. (in Chinese with English abstract) DOI:10.7506/spkx1002-6630-20181013-105. http://www.spkx.net.cn