随着人们生活节奏日益加快,精神压力持续增加,抑郁症的发生率逐年上升。世界卫生组织统计显示全球约有2.8亿 人患有抑郁症,而在中国罹患抑郁障碍的有近1亿 人,成人抑郁障碍的终身患病率为6.8%,且总治疗率不足1%[1]。抑郁症是一种常见的精神疾病,主要表现为情绪低落、沮丧、食欲不振、失眠、个体无价值感等,是精神科自杀率最高的疾病。世界卫生大会曾多次讨论精神卫生问题,并于2019年批准将世界卫生组织的《全面心理健康行动计划》延长至2030年。根据患者抑郁发作的严重程度和类型,治疗手段包括心理治疗,如行为激活、认知行为疗法和人际心理疗法,或药物治疗,如选择性血清素再摄取抑制剂和三环类抗抑郁药等。但是目前治疗抑郁的药物除了在长期治疗的过程中显示出一些效果外,还会伴随一系列副作用,如体重增加、低血压、性功能障碍和抗胆碱作用[2]。因此寻找途径缓解抑郁症状的同时减少副作用对抑郁症的改善与治疗具有十分重要的意义。多项研究表明,存在于植物中的多种天然化合物可发挥抗抑郁作用,如黄酮类化合物[3]、多糖[4]、类胡萝卜素[5]、生物碱[6]、植物激素[7]等。
人体肠道菌群能有效地转化各种食物和外源物质,与人类生理健康有着广泛的联系,在先天和适应性免疫反应的发展和功能[8-9]、肠道运动调节[10]、营养吸收[11]以及脂肪分布等方面发挥着关键作用,被认为是人体的重要“器官”[12-13]。近些年来肠道菌群与人类大脑和行为之间的相互影响被广泛研究,许多潜在的分子机制也被逐渐阐明,多项研究表明,肠道菌群组成的改变与精神疾病(如抑郁、焦虑)的发生具有重要关联[14-15]。肠道菌群的代谢产物,包括5-羟色胺(5-hydroxytryptamine,5-HT)[16]、多巴胺(dopamine,DA)[17]、去甲肾上腺素(norepinephrine,NE)[18]、短链脂肪酸(short-chain fatty acids,SCFAs)[19]、γ-氨基丁酸(γ-aminobutyric acid,GABA)[20]、组胺[21]和乙酰胆碱[22]等,可以通过不同途径影响中枢神经系统,包括免疫系统、肠脑神经系统和内分泌系统[23],同时还能通过微生物-肠-脑(the microbiota-gut-brain,MGB)轴对人的情绪产生影响。
黄酮类化合物普遍分布于水果和蔬菜中,与人体的生命活动与疾病息息相关。黄酮类化合物在神经药理学上一直以来备受关注,其药理作用主要包括降低神经元损伤、减少神经炎症发生,以及提高认知和记忆力等[24-25]。对人体肠道菌群的研究表明,黄酮类化合物能通过调节肠道菌群的结构和丰度从而对机体的生理功能产生影响,如葡萄糖稳态、脂质和能量代谢、心血管疾病以及抑郁症等[26-27]。本文将从肠道菌群角度阐述黄酮类化合物通过MGB轴发挥抗抑郁作用研究进展,为膳食类黄酮指导人体健康提供理论支撑。
1 肠道菌群与抑郁症之间的关系
1.1 抑郁症患者体内肠道菌群的组成变化
人体肠道菌群主要包括厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidetes)、变形菌门(Proteobacteria)、放线菌门(Actinobacteria)、疣微菌门(Verrucomicrobia)、梭杆菌门(Fusobacteria)6大类。肠道菌群对人体健康非常重要,其组成、数量和种类的不平衡可导致各种人体疾病,如肥胖、2型糖尿病、肠道疾病及心血管疾病等[28],影响宿主生理调节。
抑郁症患者的肠道菌群组成与健康对照组有显著差异,主要表现为厚壁菌门、放线菌门和拟杆菌门这3 类菌群发生改变[29],尤其是在重度抑郁症患者中拟杆菌门/厚壁菌门的比例增加[30]。Radjabzadeh等[31]调查研究了荷兰鹿特丹研究队列中1054 名参与者的粪便菌群多样性及组成与抑郁症之间的关系,并在一个阿姆斯特丹队列的1539 名受试者中验证了这些发现,结果鉴定出肠道菌群属水平上13 种细菌与抑郁症有关联,这些细菌参与了谷氨酸、丁酸、5-HT和GABA的合成。Liu Lanxiang等[32]对抑郁症发病机制中涉及的肠道菌群变化进行全面概述并评估了抑郁症患者、啮齿动物和非人灵长类动物抑郁症模型中物种特征微生物的变化,总结出抑郁症患者体内肠道菌群β-多样性存在显著差异,其肠道群落平衡被破坏且肠道功能改变,特征变化是致病菌属(如脱硫弧菌属和大肠杆菌志贺菌属)呈现增加趋势,有益细菌属(如双歧杆菌和粪杆菌)呈减少趋势。这些细菌也与抑郁症的严重程度有关,表明抑郁症患者的肠道菌群变化特征是促炎细菌富集和产丁酸的抗炎细菌减少。
1.2 肠道菌群参与调节抑郁症的机制
胃肠道中的细菌,包括共生菌、益生菌和致病菌,可以通过MGB轴涉及的免疫、内分泌和神经系统3 种途径与宿主相互作用,实验动物模型和临床前证据表明,肠道菌群可以利用这种双向通信网络调节大脑的发育、功能甚至行为[29,33-34]。MGB轴通过免疫系统激活(如炎症细胞因子)、神经递质(如5-HT、GABA等)及肠道菌群代谢物(SCFAs和关键膳食氨基酸,如丁酸和色氨酸)的产生,在调节情绪、行为和大脑神经元传输方面发挥重要作用[35]。下文介绍肠道菌群通过调节炎症反应、神经递质、SCFAs、下丘脑-垂体-肾上腺(the hypothalamic-pituitary-adrenal,HPA)轴途径与大脑相互作用调节抑郁症的机制。
1.2.1 炎症反应
肠道菌群通过调节炎症反应参与免疫系统的双向传递。MGB轴上双向交流的炎症信号影响宿主的健康状态,刺激调节免疫反应,帮助恢复体内稳态平衡或放大炎症[36]。
压力会促进神经炎症,从而引发抑郁症状[37]。外周炎症可以通过多种途径向大脑发出信号并导致严重抑郁症,如迷走神经、血脑屏障和细胞因子转运系统[38]。树突状细胞和单核细胞构成先天免疫反应的第一层,嵌入胃肠道相关淋巴组织,肠道菌群及其代谢物可以通过影响树突状细胞和单核细胞的活性进一步影响免疫反应[39]。特定的肠道菌群代谢产物还会改变促炎T辅助细胞17(T helper 17,Th17)与抗炎调节性T细胞(regulatory T cell,Treg)的比例[40-42],Th17与Treg细胞的失衡与抑郁症的发展密切相关[43]。此外,神经炎症发生与神经胶质细胞密切相关,它们与肠道菌群之间相互影响。小胶质细胞是中枢神经系统的主要免疫细胞,具有呈递抗原能力,并与淋巴细胞合作以限制病原体入侵。肠道菌群与小胶质细胞具有重要联系,可以影响小胶质细胞的形态、成熟、表型和功能[44-45]。抑郁症被认为是炎症状态下的小胶质细胞激活或小胶质细胞衰退和衰老(如衰老或慢性不可预知的压力)导致的小胶质细胞稳态偏离[46]。星形胶质细胞是中枢神经系统中最丰富的神经胶质细胞,具有促进血脑屏障完整性和参与免疫调节的关键功能[36]。研究表明,肠道菌群在减少星形胶质细胞活化中起关键作用,并可能缓解脑部疾病的症状[47]。肠道生态失调导致肠道通透性先于中枢神经系统免疫改变并诱发神经炎症[48],直接体现了炎症信号通过MGB轴的双向交流对生理行为的调节以及炎症相关疾病的病理至关重要,影响着抑郁症的发生发展。
另一方面,炎症伴随氧化和亚硝化应激的增加,可能在抑郁症发病机制中发挥关键作用[49-50],在抑郁症患者中表现为脂质过氧化增加和线粒体活性氧产生增加[51-52]。活性氧自由基由炎症细胞产生,并可启动细胞内信号传导,导致促炎基因的表达,进而会造成神经细胞的功能障碍。促炎细胞因子(如肿瘤坏死因子-α(tumor necrosis factor α,TNF-α)、白细胞介素(interleukin,IL)-1α、IL-1β、IL-4、IL-5、IL-6、IL-12和干扰素-γ等)的增加与抗氧化酶活性减弱、抗氧化水平降低有关,而炎症标志物的过度产生与抑郁症的认知变化相关[53]。肠道源性炎症因子的全身释放可改变血脑屏障的完整性并导致大脑发育缺陷,对健康个体的实验研究结果证实,TNF-α和IL-6可诱导抑郁情绪、焦虑和记忆和注意力受损[54]。研究发现,与健康对照组相比,重度抑郁症患者血液样本中的诱导性一氧化氮合酶表达更高,且氧化应激水平、脂多糖(lipopolysaccharide,LPS)水平更高;与此同时,重度抑郁症患者肠道菌群中嗜胆菌属和另枝菌属的比例较高,而丁酸弧菌属和戴阿利斯特杆菌属的水平较低[55]。过量分泌的促炎细胞因子可通过过度激活HPA轴、影响单胺递质含量、氧化应激损害神经细胞。有研究表明在抑郁症患者的肠道菌群中,脱硫弧菌属的丰度显著增加[56],脱硫弧菌属可产生破坏肠道屏障的LPS,导致炎症因子的产生,可破坏肠道上皮细胞,并通过血液循环对大脑额叶皮层和海马区产生影响,从而导致抑郁[57-58],在整个过程中MGB轴发挥了重要作用。拟杆菌科、普氏菌科具有抗炎作用,对外界刺激更为敏感,研究表明这两种肠道菌群丰度的下降与抑郁相关,可能是抑郁症的潜在生物标志物[59]。
1.2.2 神经递质
神经递质是一种化学物质,用于将信息传递到大脑的不同部分,在MGB轴信号传导和大脑神经网络中发挥重要作用。一些肠道菌群能够产生与动物神经系统中发现的相同的神经调节物质,包括5-HT、NE、DA、GABA和乙酰胆碱[18],这些化合物能通过小肠上皮进入全身循环,其中一些可以通过血脑屏障直接作用于大脑[60],在调节情绪、食欲和胃肠道运动方面有重要作用。
广泛分布于全身的5-HT是MGB轴上的一个关键信号分子[61-62],肠道提供了全身约95%的5-HT[16]。5-HT又名血清素,是一种重要的神经递质,主要由胃肠道的肠嗜铬细胞合成[63],在肠神经系统中作为神经递质和局部激素,能产生愉悦情绪。肠道菌群可以通过影响中枢神经系统合成5-HT所需的色氨酸代谢调节大脑功能[64],通过肠道吸收后,色氨酸可穿过血脑屏障,参与5-HT的合成[65]。NE系统是重要的弥散性神经递质系统,与抑郁症的密切联系主要体现在对中枢神经的投射,调节个体的动机、专注力和运动性等[66]。DA由中枢神经系统分泌,与快乐、兴奋等情绪相关,并有助于记忆力的提升,在功能方面与抑郁症有交集。由于大脑内侧前额叶皮质中DA能神经元在海马额叶皮质突触传递的增强调控中起关键作用,DA能神经元损伤也会损害海马-额叶皮质突触可塑性,导致认知障碍[67]。
研究表明,经微生物治疗后,与对照组相比,在肠道菌群改变的啮齿动物中,5-HT、DA和NE及其各自的前体、代谢物或受体的水平在不同的大脑区域有显著差异[68]。Li Huawei等[69]探索调节肠道菌群对5-HT代谢的影响,结果显示益生元和益生菌治疗后大鼠结肠5-HT降低,额叶皮质和海马中5-HT升高,表明肠道菌群的紊乱与5-HT代谢的变化有因果关系。研究表明,选择性血清素再摄取抑制剂氟西汀对鼠李糖乳杆菌和大肠杆菌有较强的体外抑菌活性;在大鼠饮用水中给药氟西汀剂量为10 mg/(kg·d)、持续4 周可有效抑制琥珀弧菌和普雷沃氏菌在盲肠的生长[70]。这些发现说明,肠道菌群中部分细菌能与宿主神经递质相互作用,以提高自身在肠道中的适应性,表明MGB轴在主导神经递质调节过程中做出对宿主有益的贡献,从而对机体情绪产生深刻影响。
1.2.3 SCFAs
SCFAs是肠道菌群的关键代谢产物,主要由乙酸、丙酸和丁酸组成,不仅影响肠道运动,还能激活迷走神经,并进入人体循环系统,被认为在神经免疫、内分泌调节中具有关键作用[71]。SCFAs在减少局部炎症、防止病原体浸润、维持大肠的正常功能和结肠上皮细胞的形态、功能等方面至关重要[72]。不仅如此,肠道菌群还能通过产生SCFAs刺激中枢神经系统和肠道影响身体神经递质水平[73-75]。SCFAs可通过穿过血脑屏障直接影响大脑,导致血脑屏障完整性、神经传递、脑源性神经营养因子(brain-derived neurotrophic factor,BDNF)和血清素生物合成的改变,对抑郁症的调控具有重要意义[58,76]。Zheng Peng等[77]对抑郁食蟹猴模型的肠道菌群组成、功能和MGB轴代谢特征进行研究,结果发现其厚壁菌门组成和MGB轴脂质代谢发生改变,进一步加权基因共表达网络分析表明肠道菌群可能通过调节脂肪酸、鞘脂和甘油磷脂代谢参与食蟹猴抑郁行为的发生。此外,Wu Min等[19]探讨了抑郁小鼠SCFAs变化与肠道菌群改变之间的关系,结果发现与对照组相比,抑郁小鼠的3 种主要SCFAs(乙酸、丙酸、戊酸)显著降低。该研究揭示了在抑郁小鼠体内,乙酸与5 种不同的细菌类群之间存在显著的正相关性,分别是变形菌纲、链球菌科、葡萄球菌属、葡萄球菌科和阿克曼菌属;而异杆菌属和螺旋体科与丙酸呈显著正相关,表明肠道菌群可能通过调节粪便样本中SCFAs的水平从而在抑郁症的发病机制中发挥重要作用。
产生SCFAs的细菌包括但不限于拟杆菌、双歧杆菌、丙酸杆菌、真杆菌、乳杆菌、梭状芽孢杆菌、玫瑰菌和普雷沃菌[78]。也有报道称粪杆菌、菊粉玫瑰菌,以及阿利斯提比斯是合成SCFAs的主要菌种[79-80]。这些肠道菌群通过产生SCFAs抑制病原体的生长,从而改变肠道pH值[81]。例如,双歧杆菌在乳糖发酵过程中降低了肠道pH值,从而防止肠道中致病性大肠杆菌的定植[82],调控肠道稳态。Burton等[83]研究了34 位参加减肥和抑郁综合行为干预的成年人抑郁和焦虑评分之间的关系,分析2 个月内粪便SCFAs、血浆中炎症性细胞因子和饮食标志物的变化以及6 个月内抑郁和焦虑评分的变化,发现其粪便中SCFAs和血浆中TNF-α的变化与抑郁和焦虑评分的变化呈正相关,即SCFAs排泄量增加和体内循环的SCFAs水平降低可能表明肠道健康受损、压力增加和炎症,说明了SCFAs与抑郁症有一定相关性,这可能是MGB轴的潜在作用结果。Hao Zikai等[84]对慢性不可预知性应激(chronic unpredictable mild stress,CUMS)大鼠给药普拉梭菌后,发现大鼠盲肠中SCFAs水平和血浆中IL-10水平显著升高,结果进一步证明肠道菌群在焦虑和抑郁中发挥关键作用,说明SCFAs作为MGB轴中关键信号分子对抑郁症的整体调控具有重要意义。
1.2.4 HPA轴
HPA轴是控制和调节应激反应的主要神经内分泌系统,其稳态的破坏与脑损伤、炎症和心血管损伤相关。大部分抑郁症患者表现出HPA轴的过度激活,从而增加了应激激素皮质醇的持续分泌以及炎症生物标志物的水平升高,这些激素调节许多过程,如发育、代谢、行为和免疫功能等[85]。目前肠道菌群与HPA轴之间的关系研究不足,仅停留在动物研究阶段。研究表明,HPA轴活性与肠道菌群组成有关,且应激相关的HPA轴反应可能会增加肠道通透性[86]。对无菌和常规小鼠的研究表明,肠道菌群,特别是共生菌群可以影响小鼠的HPA轴对应激的反应[87],用益生元和益生菌治疗会减弱HPA的应激反应[88],体现了肠道菌群可以通过影响机体激素分泌而参与HPA轴机制对抑郁症进行干预。
2 黄酮类化合物通过肠道菌群改善抑郁症的机制
黄酮类化合物及其代谢物不仅能直接作用于人体组织,还能调节肠道菌群的数量和类型,增加肠道菌群丰度,维持肠道微生态平衡[89-90]。
黄酮类化合物调节肠道菌群的机制主要包括两个方面:一方面,黄酮类化合物通过破坏有害菌的细胞膜中脂质双分子层或影响其细胞膜的通透性等方式,抑制肠道中大肠杆菌、金黄色葡萄球菌等有害菌群的生长[91],导致菌群分布的变化,降低致病菌对机体的生物毒性;另一方面,黄酮类化合物为肠道菌群提供代谢底物,促进双歧杆菌、乳酸杆菌等有益菌群的生长,从而优化肠道菌群结构,维持肠道菌群的稳定,并且这种代谢会产生可被吸收的更小分子,进入循环,能够到达远端器官[92],对健康产生积极意义[28,93-94]。黄酮类化合物可提高大脑中的神经递质水平、减轻炎症反应、调节肠道菌群及其代谢物(如SCFAs等),不仅影响中枢神经的发育和免疫屏障,还能通过调控MGB轴改善抑郁症[95-96]。图1反映了基于MGB轴的黄酮类化合物抗抑郁作用机制。表1总结了部分常见的黄酮类化合物调节肠道菌群并发挥抗抑郁作用的机制。
表1 常见的黄酮类化合物通过调节肠道菌群抗抑郁机制
Table 1 Antidepressant mechanisms of common flavonoids by regulating the gut microbiota

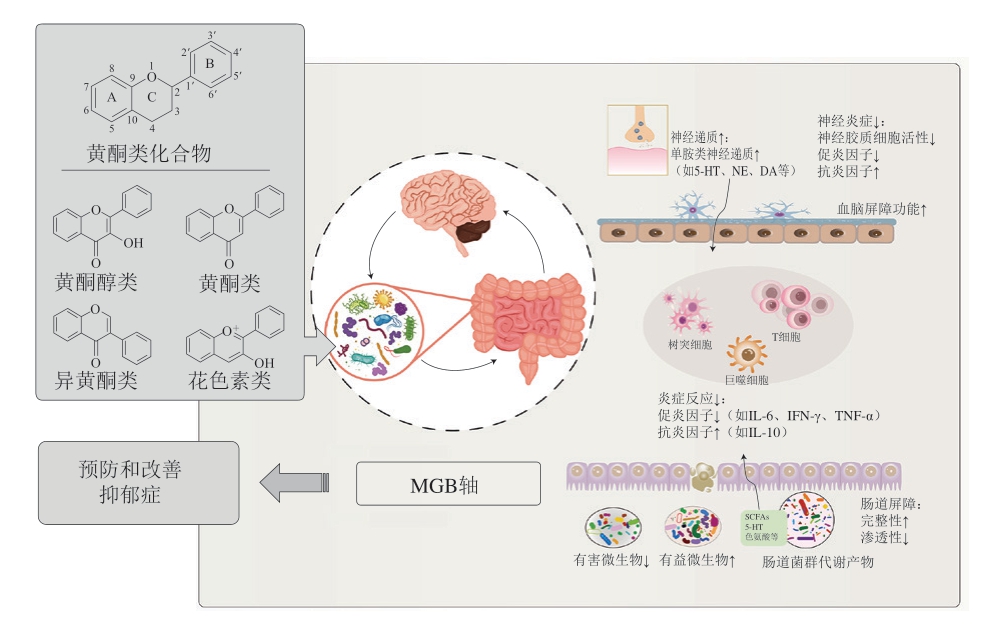
图1 基于MGB轴的黄酮类化合物抗抑郁作用机制
Fig.1 Mechanisms for the antidepressant effect of flavonoids based on the MGB axis
2.1 黄酮类化合物对炎症因子的调节
促炎细胞因子与一系列疾病行为模式有关,包括情绪抑郁、嗜睡、睡眠和食欲障碍等,所有这些都是抑郁症的特征[105]。据报道,在抑郁症患者中,包括IL-1、IL-6、TNF-α、IL-8和IL-12在内的促炎细胞因子水平升高[106-107]。研究人员发现免疫介导炎症性疾病常见的几种不同丰度模式的肠道菌群,包括乳杆菌、双歧杆菌和粪杆菌在内的几个属已被证明可以刺激包括IL-10在内的抗炎细胞因子[108],并下调促炎细胞因子[109]。
黄酮类化合物可以调节肠道中与炎症相关的细胞和菌群的组成,具有明显的抗炎特性[110]。黄酮醇被认为是治疗抑郁症有效且安全的选择,在很大程度上是基于其显著的抗氧化和抗炎作用,研究表明它们能够恢复HPA轴的神经内分泌水平,促进神经发生,并减轻抑郁行为[111]。Bijani等[112]研究了芹菜素对链脲佐菌素诱导的抑郁小鼠的影响,结果表明给药20 mg/kg的芹菜素可以调节抑郁动物的行为功能障碍、炎症标志物和恢复细胞抗氧化水平,说明其是治疗抑郁症的一种合适的候选药物。Pereira等[113]对慢性轻度应激模型小鼠腹腔内持续14 d给药杨梅素10 mg/kg,结果发现模型小鼠的抑郁行为得到显著改善,且应激小鼠的海马IL-6水平降低,这种抗抑郁作用与其抗炎活性相关。Wang Rong等[102]研究表明应激使小鼠体内炎症因子表达水平升高,而黄芪总黄酮能够有效改善肠道炎症,抑制炎症因子(IL-6、IL-1β和TNF-α)的表达水平,通过增加另枝菌属的丰度改善应激诱导的肠道菌群紊乱和肠道屏障障碍的情况。Gao等[103]研究表明富含木犀草素的花生壳提取物通过降低CUMS小鼠肠组织中的炎症标志物(IL-1β、IL-6、TNF-α和LPS)从而减轻抑郁小鼠的肠道炎症,并修复紧密连接蛋白的完整性,可通过调节肠道菌群促进肠道上皮屏障的稳定性。
2.2 黄酮类化合物对神经递质的调节作用
肠道菌群可以合成并释放多种神经递质,如GABA、NE、DA和5-HT等[114],这些神经递质是肠道菌群和大脑之间的神经调节剂[115],与抑郁症的形成和改善密切相关,且5-HT和NE也是大多数抗抑郁药物的靶点。Wang Chao等[116]综述了黄酮类化合物对GABA、5-HT、DA、BDNF等神经递质的调节作用,表明其与抑郁症的缓解具有极强的相关性。研究表明5,7-二羟基黄酮有多种神经药理活动,包括神经递质系统(GABA、5-HT、DA和NE)、神经营养因子(如BDNF和神经生长因子)的激活,其发挥抗抑郁作用是通过与特定的神经递质系统相互作用,从而产生GABA和5-HT[117]。Fang Jili等[118]研究了紫色花椰菜的花青素对CUMS小鼠抑郁模型的影响及其机制,结果显示给药小鼠抑郁行为发生明显改变,其作用机制在于通过抑制单胺氧化酶活性增加单胺类神经递质含量,增加海马BDNF的表达。Wang Li等[98]以CUMS诱导的抑郁大鼠为模型,研究大豆异黄酮(soy isoflavones,SI)减轻抑郁行为的作用机制,结果显示特定剂量的SI显著调节了肠道菌群的组成,且单胺类神经递质水平增加,主要表现为抑郁小鼠体内厚壁菌门与拟杆菌门的比例恢复到正常水平;丹毒科和双歧杆菌科的相对丰度降低;大鼠肠道多样性恢复。也就是说,SI可能通过重塑肠道菌群的结构影响CUMS大鼠的单胺类神经递质,从而改善抑郁行为。
2.3 黄酮类化合物对SCFAs的调节作用
SCFAs具有免疫调节特性,可通过刺激交感神经和自主神经系统与神经细胞相互作用,在维持血脑屏障完整性方面发挥着重要作用[119]。黄酮类化合物对肠道中SCFAs的产生有潜在影响,而SCFAs可能穿过血脑屏障直接影响大脑,导致血脑屏障完整性、神经传递、神经营养因子和血清素生物合成的改变,在调节大脑情绪方面有重要意义[58,76]。Hou Qihang等[120]研究表明,染料木素介导的肠道菌群发生显著改变,而且能够增加SCFAs的产生,并进一步通过降低肠道炎症和通透性、增加上皮细胞再生改善肠道屏障功能。木犀草素能够抑制致病菌、促进SCFAs的产生和抑制α-葡萄糖苷酶活性,从而改善肠道生态失调[121]。Peng Luyuan等[122]研究了黄芩苷对肠道菌群的调节作用,结果表明黄芩苷能够增加产生SCFAs细菌的丰度,从而增加包括乙酸、丙酸和丁酸在内的SCFAs的产生,重塑肠道菌群失调。Xiao Huihui等[123]研究了富含异黄酮的刺桐皮提取物与肠道菌群的相互作用,结果表明该提取物可以调节肠道菌群多样性,并增加体内SCFAs的循环。Jin Chengni等[124]研究表明天然黄酮类化合物蒙花苷增加了能产生SCFAs的细菌的相对丰度,包括乳杆菌、玫瑰菌、副杆菌和蓝杆菌,并提高SCFAs的含量。虽然关于黄酮类化合物通过调节肠道菌群对SCFAs的代谢从而改善抑郁症的研究不足,但是种种证据表明SCFAs能够改善抑郁情绪,且黄酮类化合物对其有重要的调节作用,这能为后续多角度探究肠道菌群与大脑之间的双向交流提供新思路。
2.4 黄酮类化合物的代谢物对抑郁症的改善作用
黄酮类化合物在肠道菌群的作用下会发生生物转化从而产生一系列代谢物,这些代谢物保留了其抗氧化和抗炎等特性,可以通过迷走神经和体循环运输到脑细胞群,在改善抑郁症中发挥重要作用[125]。槲皮素代谢物槲皮素3-葡萄糖醛酸苷抑制了小鼠脑线粒体单胺氧化酶-A与5-HT反应产生的过氧化氢,有效调节5-HT的活性从而改善抑郁症[126]。S-雌马酚是膳食大豆异黄酮的主要代谢产物,可抑制星形胶质细胞中脂多糖刺激的神经炎症,使单胺类神经递质水平正常化、逆转色氨酸代谢功能障碍、增强突触可塑性从而发挥改善抑郁症的作用[127]。黄酮类化合物的代谢物原儿茶酸具有抗抑郁活性,它能通过调节神经递质和降低海马和大脑皮层中促炎因子的表达而改善大鼠抑郁行为[128]。
3 结语
近年来,肠道菌群与大脑功能之间的双向交流在抑郁动物模型和人类临床研究中得到了广泛的探讨。虽然关于MGB轴的研究还处于相对初级阶段,其具体作用途径及机制也尚未被完全阐明,但是随着研究的不断深入,微生物、肠道、大脑三者之间的相互作用逐渐显现。肠道菌群可通过MGB轴参与调节免疫炎症性反应、神经递质分泌、SCFAs和激素产生等,在抑郁症的发生和发展过程中发挥重要作用。作为一类天然、安全的植物化合物,黄酮类化合物可通过调节肠道菌群的种类、丰度及代谢,多途径影响MGB轴中的某些信号传导通路,从而发挥抑制炎症反应、增加神经递质分泌、促进SCFAs合成等作用,最终达到预防和改善抑郁症的效果。鉴于肠道菌群对人体生理健康有着深刻的影响,从肠道菌群的角度探究抑郁症的发病机制,研究抑郁症不同阶段肠道菌群的变化与抑郁行为和认知功能的关系,能够为后续改善和治疗抑郁症提供新思路。未来对MGB轴的调控有望成为一种可控的调节手段,可通过益生菌干预、粪菌移植等方法,恢复肠道菌群的稳态和平衡,从而改善精神疾病,助力人类健康发展。
[1]LU J,XU X F,HUANG Y Q,et al.Prevalence of depressive disorders and treatment in china: a cross-sectional epidemiological study[J].Lancet Psychiatry,2021,8(11): 981-990.DOI:10.1016/S2215-0366(21)00251-0.
[2]ZHOU N,GU X Y,ZHUANG T X,et al.Gut microbiota: a pivotal hub for polyphenols as antidepressants[J].Journal of Agricultural and Food Chemistry,2020,68(22): 6007-6020.DOI:10.1021/acs.jafc.0c01461.
[3]JIA S Q,HOU Y L,WANG D,et al.Flavonoids for depression and anxiety: a systematic review and meta-analysis[J].Critical Reviews in Food Science and Nutrition,2023,63(27): 8839-8849.DOI:10.1080/10408398.2022.2057914.
[4]LI H R,XIAO Y H,HAN L,et al.Ganoderma lucidum polysaccharides ameliorated depression-like behaviors in the chronic social defeat stress depression model via modulation of Dectin-1 and the innate immune system[J].Brain Research Bulletin,2021,171: 16-24.DOI:10.1016/j.brainresbull.2021.03.002.
[5]TAO W W,RUAN J,WU R Y,et al.A natural carotenoid crocin exerts antidepressant action by promoting adult hippocampal neurogenesis through Wnt/β-catenin signaling[J].Journal of Advanced Research,2022,43: 219-231.DOI:10.1016/j.jare.2022.02.015.
[6]ZHANG M,LI A Q,YANG Q F,et al.Beneficial effect of alkaloids from Sophora alopecuroides L.on CUMS-induced depression model mice via modulating gut microbiota[J].Frontiers in Cellular and Infection Microbiology,2021,11: 665159.DOI:10.3389/fcimb.2021.665159.
[7]TANIGUTI E H,FERREIRA Y S,STUPP I J V,et al.Neuroprotective effect of melatonin against lipopolysaccharide-induced depressivelike behavior in mice[J].Physiology &Behavior,2018,188: 270-275.DOI:10.1016/j.physbeh.2018.02.034.
[8]ROUND J L,O’CONNELL R M,MAZMANIAN S K.Coordination of tolerogenic immune responses by the commensal microbiota[J].Journal of autoimmunity,2010,34(3): J220-J225.DOI:10.1016/j.jaut.2009.11.007.
[9]OLSZAK T,AN D,ZEISSIG S,et al.Microbial exposure during early life has persistent effects on natural killer T cell function[J].Science,2012,336: 489-493.DOI:10.1126/science.1219328.
[10]SAMPSON T R,DEBELIUS J W,THRON T,et al.Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease[J].Cell,2016,167(6): 1469-1480.DOI:10.1016/j.cell.2016.11.018.
[11]MILLION M,DIALLO A,RAOULT D.Gut microbiota and malnutrition[J].Microbial Pathogenesis,2017,106: 127-138.DOI:10.1016/j.micpath.2016.02.003.
[12]VALDES A M,WALTER J,SEGAL E,et al.Role of the gut microbiota in nutrition and health[J].BMJ,2018,361: 36-44.DOI:10.1136/bmj.k2179.
[13]ZHANG C G,GONG W J,LI Z H,et al.Research progress of gut flora in improving human wellness[J].Food Science and Human Wellness,2019,8(2): 102-105.DOI:10.1016/j.fshw.2019.03.007.
[14]BRUCE-KELLER A J,SALBAUM J M,BERTHOUD H R.Harnessing gut microbes for mental health: getting from here to there[J].Biological Psychiatry,2018,83(3): 214-223.DOI:10.1016/j.biopsych.2017.08.014.
[15]WINTER G,HART R A,CHARLESWORTH R P G,et al.Gut microbiome and depression: what we know and what we need to know[J].Reviews in the Neurosciences,2018,29(6): 629-643.DOI:10.1515/revneuro-2017-0072.
[16]ERSPAMER V.Pharmacology of indole-alkylamines[J].Pharmacological Reviews,1954,6(4): 425-487.
[17]TSAVKELOVA E A,BOTVINKO I V,KUDRIN V S,et al.Detection of neurotransmitter amines in microorganisms with the use of highperformance liquid chromatography[J].Doklady Biochemistry and Biophysics,2000,372: 115-117.
[18]GONZÁLEZ-ARANCIBIA C,URRUTIA-PIÑONES J,ILLANESGONZÁLEZ J,et al.Do your gut microbes affect your brain dopamine?[J].Psychopharmacology,2019,236(5): 1611-1622.DOI:10.1007/s00213-019-05265-5.
[19]WU M,TIAN T,MAO Q,et al.Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice[J].Translational Psychiatry,2020,10(1): 350.DOI:10.1038/s41398-020-01038-3.
[20]BARRETT E,ROSS R P,O’TOOLE P W,et al.γ-Aminobutyric acid production by culturable bacteria from the human intestine[J].Journal of Applied Microbiology,2012,113(2): 411-417.DOI:10.1111/j.1365-2672.2012.05344.x.
[21]THOMAS C M,HONG T,VAN PIJKEREN J P,et al.Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling[J].PLoS ONE,2012,7(2):e31951.DOI:10.1371/journal.pone.0031951.
[22]STEPHENSON M,ROWATT E,HARRISON K.The production of acetylcholine by a strain of Lactobacillus plantarum with an addendum on the isolation of acetylcholine as a salt of hexanitrodiphenylamine[J].Microbiology,1947,1(3): 279-298.DOI:10.1099/00221287-1-3-279.
[23]SONG X R,WANG L Y,LIU Y N,et al.The gut microbiota-brain axis: role of the gut microbial metabolites of dietary food in obesity[J].Food Research International,2022,153: 110971.DOI:10.1016/j.foodres.2022.110971.
[24]SPENCER J P E.Flavonoids and brain health: multiple effects underpinned by common mechanisms[J].Genes &Nutrition,2009,4(4): 243-250.DOI:10.1007/s12263-009-0136-3.
[25]MARTINS J,BRIJESH S.Phytochemistry and pharmacology of anti-depressant medicinal plants: a review[J].Biomedicine &Pharmacotherapy,2018,104: 343-365.DOI:10.1016/j.biopha.2018.05.044.
[26]OTEIZA P I,FRAGA C G,MILLS D A,et al.Flavonoids and the gastrointestinal tract: local and systemic effects[J].Molecular Aspects of Medicine,2018,61: 41-49.DOI:10.1016/j.mam.2018.01.001.
[27]SHI R Y,HUANG C Y,GAO Y,et al.Gut microbiota axis: potential target of phytochemicals from plant-based foods[J].Food Science and Human Wellness,2023,12(5): 1409-1426.DOI:10.1016/j.fshw.2023.02.001.
[28]李涛,李绮丽,张群,等.基于肠道菌群的黄酮类化合物生理功能研究进展[J].中国食品学报,2022,22(2): 357-368.DOI:10.16429/j.1009-7848.2022.02.038.
[29]DINAN T G,CRYAN J F.Brain-gut-microbiota axis: mood,metabolism and behaviour[J].Nature Reviews Gastroenterology &Hepatology,2017,14(2): 69-70.DOI:10.1038/nrgastro.2016.200.
[30]LIU L X,WANG H Y ZHANG H P,et al.Gut microbiota and its metabolites in depression: from pathogenesis to treatment[J].Ebiomedicine,2022,9(35): e2203707.DOI:10.1002/advs.202203707.
[31]RADJABZADEH D,BOSCH J A,UITTERLINDEN A G,et al.Gut microbiome-wide association study of depressive symptoms[J].Nature Communications,2022,13(1): 7128.DOI:10.1038/s41467-022-34502-3.
[32]LIU L X,WANG H Y,ZHANG H P,et al.Toward a deeper understanding of gut microbiome in depression: the promise of clinical applicability[J].Advanced Science,2022,9(35): e2203707.DOI:10.1002/advs.202203707.
[33]COLLINS S M,SURETTE M,BERCIK P.The Interplay between the intestinal microbiota and the brain[J].Nature Reviews Microbiology,2012,10(11): 735-742.DOI:10.1038/nrmicro2876.
[34]CRYAN J F,DINAN T G.Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour[J].Nature Reviews Microbiology,2012,13(10): 701-712.DOI:10.1038/nrn3346.
[35]SANDHU K V,SHERWIN E,SCHELLEKENS H,et al.Feeding the microbiota-gut-brain axis: diet,microbiome,and neuropsychiatry[J].Translational Research,2017,179: 223-244.DOI:10.1016/j.trsl.2016.10.002.
[36]AGIRMAN G,YU K B,HSIAO E Y.Signaling inflammation across the gut-brain axis[J].Science,2021,374: 1087-1092.DOI:10.1126/science.abi6087.
[37]KIM Y K,WON E.The influence of stress on neuroinflammation and alterations in brain structure and function in major depressive disorder[J].Behavioural Brain Research,2017,329: 6-11.DOI:10.1016/j.bbr.2017.04.020.
[38]QUAN N,BANKS W A.Brain-immune communication pathways[J].Brain,Behavior,and Immunity,2007,21(6): 727-735.DOI:10.1016/j.bbi.2007.05.005.
[39]WESTFALL S,CARACCI F,ZHAO D,et al.Microbiota metabolites modulate the T helper 17 to regulatory T cell (TH17/Treg) imbalance promoting resilience to stress-induced anxiety-and depressive-like behaviors[J].Brain,Behavior,and Immunity,2021,91: 350-368.DOI:10.1016/j.bbi.2020.10.013.
[40]ARPAIA N,CAMPBELL C,FAN X,et al.Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation[J].Nature,2013,504: 451-455.DOI:10.1038/nature12726.
[41]LUO A,LEACH S T,BARRES R,et al.The microbiota and epigenetic regulation of T helper 17/regulatory T cells: in search of a balanced immune system[J].Frontiers in Immunology,2017,8: 1-14.DOI:10.3389/fimmu.2017.00417.
[42]CUI H T,CAI Y Z,WANG L,et al.Berberine regulates Treg/Th17 balance to treat ulcerative colitis through modulating the gut microbiota in the colon[J].Frontiers in Pharmacology,2018,9: 571.DOI:10.3389/fphar.2018.00571.
[43]HONG M,ZHENG J,DING Z Y,et al.Imbalance between Th17 and Treg Cells may play an important role in the development of chronic unpredictable mild stress-induced depression in mice[J].Neuroimmunomodulation,2013,20(1): 39-50.DOI:10.1159/000343100.
[44]LIU L C,TONG F,LI H H,et al.Maturation,morphology,and function: the decisive role of intestinal flora on microglia: a review[J].Journal of Integrative Neuroscience,2023,22(3): 70.DOI:10.31083/j.jin2203070.
[45]DE WEERTH C.Do Bacteria shape our development? crosstalk between intestinal microbiota and HPA axis[J].Neuroscience &Biobehavioral Reviews,2017,83: 458-471.DOI:10.1016/j.neubiorev.2017.09.016.
[46]YIRMIYA R,RIMMERMAN N,RESHEF R.Depression as a microglial disease[J].Trends in Neurosciences,2015,38(10): 637-658.DOI:10.1016/j.tins.2015.08.001.
[47]ZHAO Y F,WEI D N,TANG Y.Gut microbiota regulate astrocytic functions in the brain: possible therapeutic consequences[J].Current Neuropharmacology,2021,19(8): 1354-1366.DOI:10.2174/1570159X19666210215123239.
[48]GOLOMB S M,GULDNER I H,ZHAO A,et al.Multi-modal singlecell analysis reveals brain immune landscape plasticity during aging and gut microbiota dysbiosis[J].Cell Reports,2020,33(9): 108438.DOI:10.1016/j.celrep.2020.108438.
[49]PASCO J A,NICHOLSON G C,WILLIAMS L J,et al.Association of high-sensitivity C-reactive protein with de novo major depression[J].The British Journal of Psychiatry,2010,197(5): 372-377.DOI:10.1192/bjp.bp.109.076430.
[50]GARDNER A,BOLES R G.Beyond the serotonin hypothesis:mitochondria,inflammation and neurodegeneration in major depression and affective spectrum disorders[J].Progress in Neuro-Psychopharmacology and Biological Psychiatry,2011,35(3): 730-743.DOI:10.1016/j.pnpbp.2010.07.030.
[51]ALCOCER-GÓMEZ E,DE MIGUEL M,CASAS-BARQUERO N,et al.NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder[J].Brain,Behavior,and Immunity,2014,36: 111-117.DOI:10.1016/j.bbi.2013.10.017.
[52]ANDERSON G,MAES M.Oxidative/nitrosative stress and immunoinflammatory pathways in depression: treatment implications[J].Current Pharmaceutical Design,2014,20(23): 3812-3847.DOI:10.2174/13816128113196660738.
[53]BHATT S,NAGAPPA A N,PATIL C R.Role of oxidative stress in depression[J].Drug Discovery Today,2020,25(7): 1270-1276.DOI:10.1016/j.drudis.2020.05.001.
[54]REICHENBERG A,YIRMIYA R,SCHULD A,et al.Cytokineassociated emotional and cognitive disturbances in humans[J].Archives of General Psychiatry,2001,58(5): 445-452.DOI:10.1001/archpsyc.58.5.445.
[55]CASO J R,MACDOWELL K S,GONZÁLEZ-PINTO A,et al.Gut microbiota,innate immune pathways,and inflammatory control mechanisms in patients with major depressive disorder[J].Translational Psychiatry,2021,11(1): 645.DOI:10.1038/s41398-021-01755-3.
[56]ZHU H Z,LIANG Y D,MA Q Y,et al.Xiaoyaosan improves depressive-like behavior in rats with chronic immobilization stress through modulation of the gut microbiota[J].Biomedicine &Pharmacotherapy,2019,112: 108621.DOI:10.1016/j.biopha.2019.108621.
[57]CHUDZIK A,ORZYŁOWSKA A,ROLA R,et al.Probiotics,prebiotics and postbiotics on mitigation of depression symptoms:modulation of the brain-gut-microbiome axis[J].Biomolecules,2021,11(7): 1000.DOI:10.3390/biom11071000.
[58]MORAIS L H,SCHREIBER H L,MAZMANIAN S K.The gut microbiota-brain axis in behaviour and brain disorders[J].Nature Reviews Microbiology,2021,19(4): 241-255.DOI:10.1038/s41579-020-00460-0.
[59]LIN S S,LI Q Q,XU Z J,et al.Detection of the role of intestinal flora and tryptophan metabolism involved in antidepressant-like actions of crocetin based on a multi-omics approach[J].Psychopharmacology,2022,239(11): 3657-3677.DOI:10.1007/s00213-022-06239-w.
[60]WANG Y,KASPER L H.The role of microbiome in central nervous system disorders[J].Brain Behavior and Immunity,2014,38: 1-12.DOI:10.1016/j.bbi.2013.12.015.
[61]BERGER M,GRAY J A,ROTH B L.The expanded biology of serotonin[J].Annual Review of Medicine,2009,60: 355-366.DOI:10.1146/annurev.med.60.042307.110802.
[62]MAWE G M,HOFFMAN J M.Serotonin signalling in the gut:functions,dysfunctions and therapeutic targets[J].Nature Reviews Gastroenterology &Hepatology,2013,10(8): 473-486.DOI:10.1038/nrgastro.2013.105.
[63]GERSHON M D,TACK J.The serotonin signaling system:from basic understanding to drug development for functional GI disorders[J].Gastroenterology,2007,132(1): 397-414.DOI:10.1053/j.gastro.2006.11.002.
[64]BEN-ARI Y.Neuropaediatric and neuroarchaeology: understanding development to correct brain disorders[J].Acta Paediatrica,2013,102(4): 331-334.DOI:10.1111/apa.12161.
[65]RUDDICK J P,EVANS A K,NUTT D J,et al.Tryptophan metabolism in the central nervous system: medical implications[J].Expert Reviews in Molecular Medicine,2006,8(20): 1-27.DOI:10.1017/S1462399406000068.
[66]UPPAL A,SINGH A,GAHTORI P,et al.Antidepressants: current strategies and future opportunities[J].Current Pharmaceutical Design,2010,16(38): 4243-4253.DOI:10.2174/138161210794519110.
[67]LAROCHE S,DAVIS S,JAY T M.Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation[J].Hippocampus,2000,10(4): 438-446.DOI:10.1002/1098-1063(2000)10:4<438::aid-hipo10>3.0.co;2-3.
[68]HUANG F,WU X J.Brain neurotransmitter modulation by gut microbiota in anxiety and depression[J].Frontiers in Cell and Developmental Biology,2021,9: 649103.DOI:10.3389/fcell.2021.649103.
[69]LI H W,WANG P,HUANG L Q,et al.Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model[J].Neurogastroenterology &Motility,2019,31(10): e13677.DOI:10.1111/nmo.13677.
[70]CUSSOTTO S,STRAIN C R,FOUHY F,et al.Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function[J].Psychopharmacology,2019,236(5): 1671-1685.DOI:10.1007/s00213-018-5006-5.
[71]YAO Y,CAI X Y,FEI W D,et al.The role of short-chain fatty acids in immunity,inflammation and metabolism[J].Critical Reviews in Food Science and Nutrition,2020,62(1): 1-12.DOI:10.1080/10408398.2020.1854675.
[72]YOO J Y,GROER M,DUTRA S V O,et al.Gut microbiota and immune system interactions[J].Microorganisms,2020,8(10): 1587.DOI:10.3390/microorganisms8101587.
[73]EVRENSEL A,CEYLAN M E.The gut-brain axis: the missing link in depression[J].Clinical Psychopharmacology and Neuroscience,2015,13(3): 239-244.DOI:10.9758/cpn.2015.13.3.239.
[74]GALLAND L.The gut microbiome and the brain[J].Journal of Medicinal Food,2014,17(12): 1261-1272.DOI:10.1089/jmf.2014.7000.
[75]SONG X R,LIU Y N,ZHANG X,et al.Role of intestinal probiotics in the modulation of lipid metabolism: implications for therapeutic treatments[J].Food Science and Human Wellness,2023,12(5): 1439-1449.DOI:10.1016/j.fshw.2023.02.005.
[76]DALILE B,VAN OUDENHOVE L,VERVLIET B,et al.The role of short-chain fatty acids in microbiota-gut-brain communication[J].Nature Reviews Gastroenterology &Hepatology,2019,16(8): 461-478.DOI:10.1038/s41575-019-0157-3.
[77]ZHENG P,WU J,ZHANG H P,et al.The gut microbiome modulates gut-brain axis glycerophospholipid metabolism in a region-specific manner in a nonhuman primate model of depression[J].Molecular Psychiatry,2020,26(6): 2380-2392.DOI:10.1038/s41380-020-0744-2.
[78]MACFARLANE G T,MACFARLANE S.Bacteria,colonic fermentation,and gastrointestinal health[J].Journal of AOAC International,2012,95(1): 50-60.DOI:10.5740/jaoacint.sge_macfarlane.
[79]TICINESI A,MANCABELLI L,TAGLIAFERRI S,et al.The gutmuscle axis in older subjects with low muscle mass and performance:a proof of concept study exploring fecal microbiota composition and function with shotgun metagenomics sequencing[J].International Journal of Molecular Sciences,2020,21(23): 8946.DOI:10.3390/ijms21238946.
[80]PARKER B J,WEARSCH P A,VELOO A C M,et al.The genus Alistipes: gut bacteria with emerging implications to inflammation,cancer,and mental health[J].Frontiers in Immunology,2020,11: 906.DOI:10.3389/fimmu.2020.00906.
[81]MASLOWSKI K M,VIEIRA A T,NG A,et al.Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43[J].Nature,2009,461: 1282-1286.DOI:10.1038/nature08530.
[82]KADAOUI K A,CORTHESY B.Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer’s patches with restriction to mucosal compartment[J].Journal of Immunology,2007,179(11): 7751-7757.DOI:10.4049/jimmunol.179.11.7751.
[83]BURTON T C J,LV N,TSAI P,et al.Associations between fecal shortchain fatty acids,plasma inflammatory cytokines,and dietary markers with depression and anxiety: post hoc analysis of the ENGAGE-2 pilot trial[J].The American Journal of Clinical Nutrition,2023,117(4): 717-730.DOI:10.1016/j.ajcnut.2023.01.018.
[84]HAO Z K,WANG W,GUO R,et al.Faecalibacterium prausnitzii(ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats[J].Psychoneuroendocrinology,2019,104: 132-142.DOI:10.1016/j.psyneuen.2019.02.025.
[85]PERRIN A J,HOROWITZ M A,ROELOFS J,et al.Glucocorticoid resistance: is it a requisite for increased cytokine production in depression? a systematic review and meta-analysis[J].Frontiers in Psychiatry,2019,10: 423.DOI:10.3389/fpsyt.2019.00423.
[86]MISIAK B,LONIEWSKI I,MARLICZ W,et al.The HPA axis dysregulation in severe mental illness: can we shift the blame to gut microbiota?[J].Progress in Neuro-Psychopharmacology and Biological Psychiatry,2020,102: 109951.DOI:10.1016/j.pnpbp.2020.109951.
[87]SUDO N,CHIDA Y,AIBA Y,et al.Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice[J].The Journal of Physiology,2004,558(1): 263-275.DOI:10.1113/jphysiol.2004.063388.
[88]ERGANG P,VAGNEROVA K,HERMANOVA P,et al.The gut microbiota affects corticosterone production in the murine small intestine[J].International Journal of Molecular Sciences,2021,22(8):4229.DOI:10.3390/ijms22084229.
[89]CHEN T T,YANG C S.Biological fates of tea polyphenols and their interactions with microbiota in the gastrointestinal tract: implications on health effects[J].Critical Reviews in Food Science and Nutrition,2020,60(16): 2691-2709.DOI:10.1080/10408398.2019.1654430.
[90]KORCZAK M,PILECKI M,GRANICA S,et al.Phytotherapy of mood disorders in the light of microbiota-gut-brain axis[J].Phytomedicine,2023,111: 154642.DOI:10.1016/j.phymed.2023.154642.
[91]TAN Z Y,DENG J,YE Q X,et al.The antibacterial activity of naturalderived flavonoids[J].Current Topics in Medicinal Chemistry,2022,22(12): 1009-1019.DOI:10.2174/1568026622666220221110506.
[92]WILLIAMSON G,CLIFFORD M N.Role of the small intestine,colon and microbiota in determining the metabolic fate of polyphenols[J].Biochemical Pharmacology,2017,139: 24-39.DOI:10.1016/j.bcp.2017.03.012.
[93]LI D,LIU R,WANG M,et al.3β-Hydroxysteroid dehydrogenase expressed by gut microbes degrades testosterone and is linked to depression in males[J].Cell Host &Microbe,2022,30(3): 329-339.DOI:10.1016/j.chom.2022.01.001.
[94]张庆建,赵毅民,杨明,等.黄酮类化合物对中枢神经系统的作用[J].中国中药杂志,2001(8): 5-8.
[95]WANG M Y,LI J Y,HU T,et al.Metabolic fate of tea polyphenols and their crosstalk with gut microbiota[J].Food Science and Human Wellness,2022,11(3): 455-466.DOI:10.1016/j.fshw.2021.12.003.
[96]POSSEMIERS S,BOLCA S,VERSTRAETE W,et al.The intestinal microbiome: a separate organ inside the body with the metabolic potential to influence the bioactivity of botanicals[J].Fitoterapia,2010,82(1): 53-66.DOI:10.1016/j.fitote.2010.07.012.
[97]LIU J H,YE T,YANG S Y,et al.Antidepressant-like activity,active components and related mechanism of Hemerocallis citrina Baroni extracts[J].Frontiers in Pharmacology,2022,13: 967670.DOI:10.3389/fphar.2022.967670.
[98]WANG L,WU X J,MA Y H,et al.Supplementation with soy isoflavones alleviates depression-like behaviour via reshaping the gut microbiota structure[J].Food &Function,2021,12(11): 4995-5006.DOI:10.1039/d0fo03254a.
[99]SONG X J,WANG W H,DING S S,et al.Puerarin ameliorates depression-like behaviors of with chronic unpredictable mild stress mice by remodeling their gut microbiota[J].Journal of Affective Disorders,2021,290: 353-363.DOI:10.1016/j.jad.2021.04.037.
[100]CHEN Z Q,CHEN X Q,ZHAI Y,et al.Effects of black rice anthocyanins on the behavior and intestinal microbiota of mice with chronic unpredictable mild stress[J].IOP Conference Series: Earth and Environmental Science,2021,792(1): 012005.DOI:10.1088/1755-1315/792/1/012005.
[101]BALASUBRAMANIAN R,BAZAZ M R,PASAM T,et al.Involvement of microbiome gut-brain axis in neuroprotective effect of quercetin in mouse model of repeated mild traumatic brain injury[J].Neuromolecular Medicine,2023,25(2): 242-254.DOI:10.1007/s12017-022-08732-z.
[102]WANG R,CHEN T,WANG Q,et al.Total flavone of abelmoschus manihot ameliorates stress-induced microbial alterations drive intestinal barrier injury in DSS colitis[J].Drug Design,Development and Therapy,2021,15: 2999-3016.DOI:10.2147/DDDT.S313150.
[103]GAO A X,XIA T C,PENG Z T,et al.The ethanolic extract of peanut shell attenuates the depressive-like behaviors of mice through modulation of inflammation and gut microbiota[J].Food Research International,2023,168: 112765.DOI:10.1016/j.foodres.2023.112765A.
[104]XIA C X,GAO A X,ZHU Y,et al.Flavonoids from seabuckthorn(Hippophae rhamnoides L.) restore CUMS-induced depressive disorder and regulate gut microbiota in mice[J].Food &Function,2023,14(16): 7426-7438.DOI:10.1039/d3fo01332d.
[105]FLUX M C,LOWRY C A.Finding intestinal fortitude: integrating the microbiome into a holistic view of depression mechanisms,treatment,and resilience[J].Neurobiology of Disease,2020,135: 104578.DOI:10.1016/j.nbd.2019.104578.
[106]DOWLATI Y,HERRMANN N,SWARDFAGER W,et al.A metaanalysis of cytokines in major depression[J].Biological Psychiatry,2010,67(5): 446-457.DOI:10.1016/j.biopsych.2009.09.033.
[107]NA K S,JUNG H Y,KIM Y K.The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia[J].Progress in Neuro-Psychopharmacology and Biological Psychiatry,2014,48: 277-286.DOI:10.1016/j.pnpbp.2012.10.022.
[108]SOKOL H,PIGNEUR B,WATTERLOT L,et al.Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients[J].Proceedings of the National Academy of Sciences,2008,105(43): 16731-16736.DOI:10.1073/pnas.0804812105.
[109]LLOPIS M,ANTOLIN M,CAROL M,et al.Lactobacillus casei downregulates commensals’ inflammatory signals in Crohn’s disease mucosa[J].Inflammatory Bowel Diseases,2009,15(2): 275-283.DOI:10.1002/ibd.20736.
[110]GIL-CARDOSO K,GINÉS I,PINENT M,et al.Effects of flavonoids on intestinal inflammation,barrier integrity and changes in gut microbiota during diet-induced obesity[J].Nutrition Research Reviews,2016,29(2): 234-248.DOI:10.1017/S0954422416000159.
[111]JAZVINŠĆAK J M,NADA O,DALIBOR K,et al.Flavonols in action: targeting oxidative stress and neuroinflammation in major depressive disorder[J].International Journal of Molecular Sciences,2023,24(8): 6888.DOI:10.3390/ijms24086888.
[112]BIJANI S,DIZAJI R,SHARAFI A,et al.Neuroprotective effect of apigenin on depressive-like behavior: mechanistic approach[J].Neurochemical Research,2022,47(3): 644-655.DOI:10.1007/s11064-021-03473-0.
[113]PEREIRA M,SIBA I P,ACCO A,et al.Myricitrin exhibits antidepressant-like effects and reduces IL-6 hippocampal levels in the chronic mild stress model[J].Behavioural Brain Research,2022,429:113905.DOI:10.1016/j.bbr.2022.113905.
[114]CLARKE G,STILLING R M,KENNEDY P J,et al.Minireview:gut microbiota: the neglected endocrine organ[J].Molecular Endocrinology,2014,28(8): 1221-1238.DOI:10.1210/me.2014-1108.
[115]SONG Z Y,CHENG L,LIU Y N,et al.Plant-derived bioactive components regulate gut microbiota to prevent depression and depressive-related neurodegenerative diseases: focus on neurotransmitters[J].Trends in Food Science &Technology,2022,129: 581-590.DOI:10.1016/J.TIFS.2022.10.019.
[116]WANG C,YANG S B,DENG J W,et al.The research progress on the anxiolytic effect of plant-derived flavonoids by regulating neurotransmitters[J].Drug Development Research,2023,84(3): 458-469.DOI:10.1002/ddr.22038.
[117]RODRÍGUEZ-LANDA J F,GERMAN-PONCIANO L J,PUGAOLGUÍN A,et al.Pharmacological,neurochemical,and behavioral mechanisms underlying the anxiolytic-and antidepressant-like effects of flavonoid chrysin[J].Molecules,2022,27(11): 3551.DOI:10.3390/molecules27113551.
[118]FANG J L,LUO Y,JIN S H,et al.Ameliorative effect of anthocyanin on depression mice by increasing monoamine neurotransmitter and upregulating BDNF expression[J].Journal of Functional Foods,2020,66(c): 103757.DOI:10.1016/j.jff.2019.103757.
[119]LOWING J L,SUSICK L L,CARUSO J P,et al.Experimental traumatic brain injury alters ethanol consumption and sensitivity[J].Journal of Neurotrauma,2014,31(20): 1700-1710.DOI:10.1089/neu.2013.3286.
[120]HOU Q H,HUANG J X,ZHAO L H,et al.Dietary genistein increases microbiota-derived short chain fatty acid levels,modulates homeostasis of the aging gut,and extends healthspan and lifespan[J].Pharmacological Research,2023,188: 106676.DOI:10.1016/j.phrs.2023.106676.
[121]DAILY J W,KANG S,PARK S.Protection against Alzheimer’s disease by luteolin: role of brain glucose regulation,anti-inflammatory activity,and the gut microbiota-liver-brain axis[J].Biofactors,2021,47(2): 218-231.DOI:10.1002/biof.1703.
[122]PENG L Y,SHI H T,TAN Y R,et al.Baicalin inhibits APEC-induced lung injury by regulating gut microbiota and SCFA production[J].Food &Function,2021,12(24): 12621-12633.DOI:10.1039/d1fo02407h.
[123]XIAO H H,YU X L,YANG C,et al.Prenylated isoflavonoidsrich extract of erythrinae cortex exerted bone protective effects by modulating gut microbial compositions and metabolites in ovariectomized rats[J].Nutrients,2021,13(9): 2943.DOI:10.3390/nu13092943.
[124]JIN C N,LIU J Y,JIN R Y,et al.Linarin ameliorates dextran sulfate sodium-induced colitis in C57BL/6J mice via the improvement of intestinal barrier,suppression of inflammatory responses and modulation of gut microbiota[J].Food &Function,2022,13(20):10574-10586.DOI:10.1039/d2fo02128e.
[125]MELROSE J.The potential of flavonoids and flavonoid metabolites in the treatment of neurodegenerative pathology in disorders of cognitive decline[J].Antioxidants,2023,12(3): 663.DOI:10.3390/antiox12030663.
[126]YOSHINO S,HARA A,SAKAKIBARA H,et al.Effect of quercetin and glucuronide metabolites on the monoamine oxidase-A reaction in mouse brain mitochondria[J].Nutrition,2011,27(7/8): 847-852.DOI:10.1016/j.nut.2010.09.002.
[127]LU C,GAO R J ZHANG Y Y,et al.S-equol,a metabolite of dietary soy isoflavones,alleviates lipopolysaccharide-induced depressivelike behavior in mice by inhibiting neuroinflammation and enhancing synaptic plasticity[J].Food &Funcion,2021,12(13): 5770-5778.DOI:10.1039/d1fo00547b.
[128]THAKARE V N,PATIL R R,SURALKAR A A,et al.Protocatechuic acid attenuate depressive-like behavior in olfactory bulbectomized rat model: behavioral and neurobiochemical investigations[J]Metabolic Brain Disease,2019,34(3): 775-787.DOI:10.1007/s11011-019-00401-8.